 TOSUN Pharmaceutical has successfully registered more than 200 APIs, excipients, and packaging materials, covering many fields such as cardiovascular and cerebrovascular, oncology, neurology, gastroenterology, and respiratory medicine. The experienced registration team, proficient in the regulatory requirements of registration, can quickly help you obtain drug registration qualifications efficiently and cost-effectively around the world. At the same time, we have more than 95% of the global API supplier resources, and can provide high-quality and stable supply.
TOSUN Pharmaceutical has successfully registered more than 200 APIs, excipients, and packaging materials, covering many fields such as cardiovascular and cerebrovascular, oncology, neurology, gastroenterology, and respiratory medicine. The experienced registration team, proficient in the regulatory requirements of registration, can quickly help you obtain drug registration qualifications efficiently and cost-effectively around the world. At the same time, we have more than 95% of the global API supplier resources, and can provide high-quality and stable supply.
With the continuous improvement of China's pharmaceutical technology and quality, the export volume of APIs in recent years has risen sharply. As a professional pharmaceutical service company based in China, we can provide you with high-quality and cheap export APIs from China.
Service Content
Review, translation, sorting and submission of DMF materials.
Registration inspection application, sample delivery, tracking and problem solving.
Tracking the whole process of API registration, issuing supplementary questions and answers and supplementary response materials for review, translation and submission.
Changes in API registration data include major changes, medium changes, minor changes and basic information changes.
Submit the annual report materials for registered APIs.
Service Process
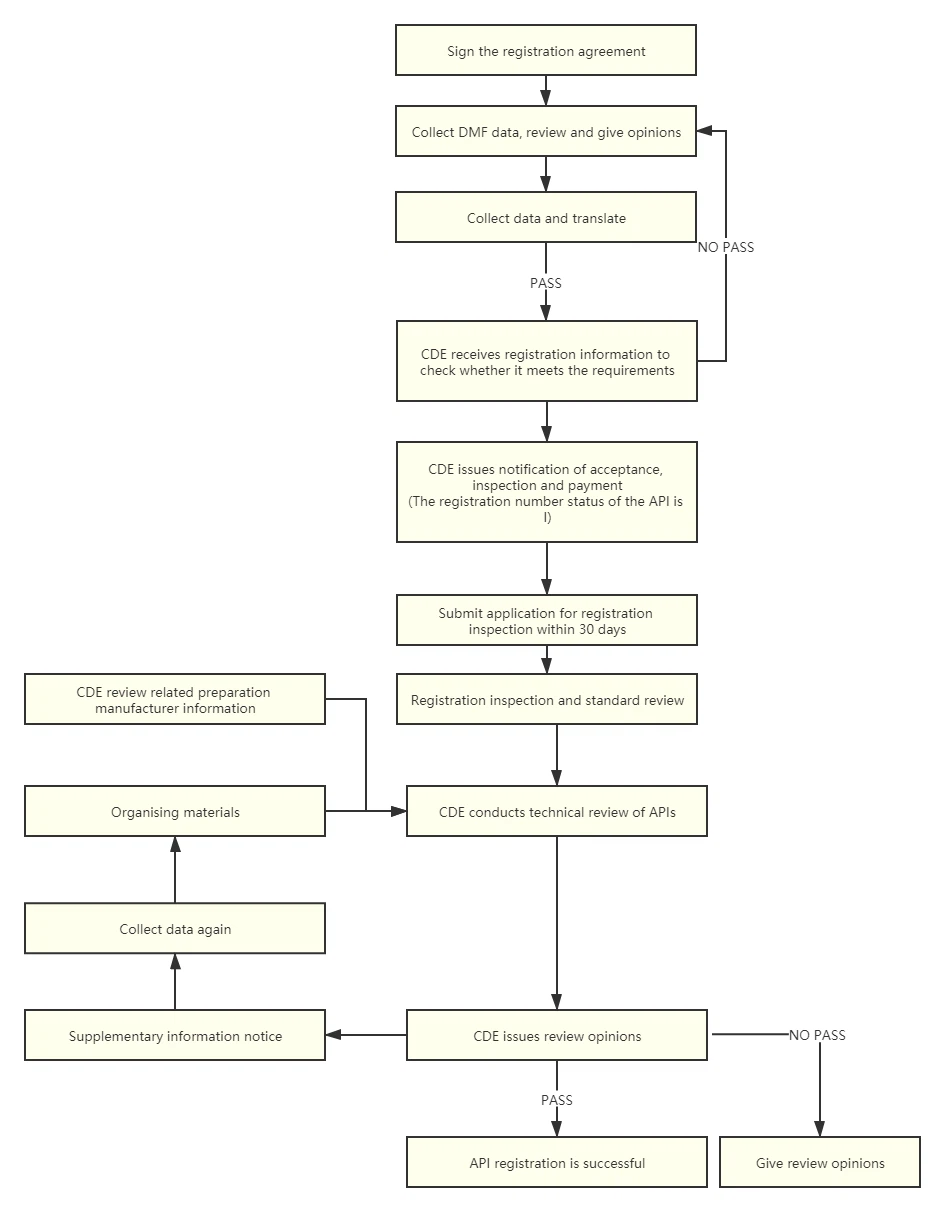
Sign an agreement to collect DMF data and documents that allow the drug to be marketed and sold.
DMF data review, request customers to supplement data based on data review.
Translation, collation and final review of DMF documents.
Submit CTD data to CDE, and CDE will conduct formal review. Need to make corrections and inadmissibility, and resubmit after completing the materials based on the supplementary opinions and reasons for inadmissibility.
After acceptance, submit the registration inspection materials and three batches of samples and reference materials for registration inspection.
CDE conducts the technical review of the API, the technical review related to the preparation or a separate review. During the review period, a supplementary notice may be issued. According to the supplementary notice, customers are required to supplement the information. After the data is submitted, CDE will continue to conduct the technical review of the API.
China drug registration process
Notes
NMPA special requirements: blank batch production records, complete process verification reports, starting materials technical documents and audit reports are required.
Proof documents: APIs need to be listed in the country of origin, customers need to provide the COPP/CEP/GMP+FSC of the APIs, and certifications are required; power of attorney and notarization documents are required; a declaration of non-infringement of API patents is required.
Registration inspection: The customer needs to provide three batches of samples and reference materials and a complete inspection report.
During the technical review process, a supplementary notice may be issued, and additional information is required as required.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China