TOSUN Pharmaceutical can provide registration agency services for your veterinary drugs exported to China.
SERVICE PROCESS
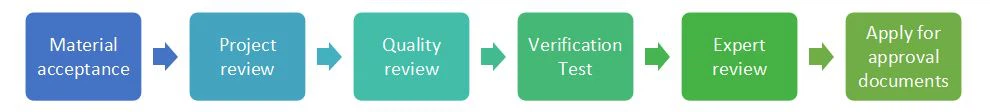
Material acceptance.
Imported medical devices should have Chinese instructions and Chinese labels. The instructions and labels shall meet the requirements of the Regulations on the Supervision and Administration of Medical Devices and the requirements of relevant mandatory standards. The instructions shall state the origin of the medical device and the name, address and contact information of the agent. No Chinese instructions, Chinese labels or instructions or labels that do not comply with the provisions of this Article shall not be imported.
Project review.
The Veterinary Bureau of the Ministry of Agriculture reviews the application materials in accordance with Chinese regulations and conducts a preliminary review.
Quality review.
The applicant sends the samples to the Veterinary Bureau of the Ministry of Agriculture for quality review based on the preliminary review opinions.
Verification Test.
The applicant conducts clinical trials of drug efficacy and related trials based on the preliminary review opinions.
Expert review.
The Veterinary Bureau of the Ministry of Agriculture conducts a final review of the application materials.
Apply for approval documents.
The Veterinary Bureau of the Ministry of Agriculture proposes an approval plan and processes the approval documents based on the review results.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China