If you have any question, please feel free to email us. We will touch with you as soon as possible.
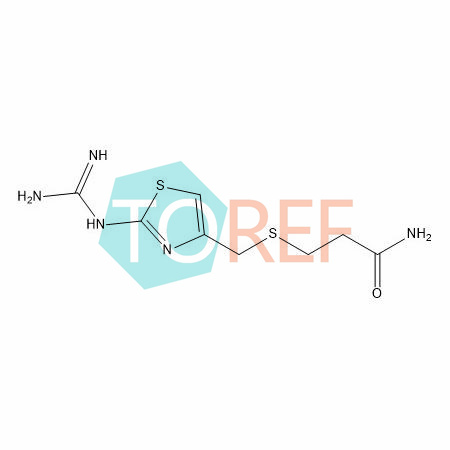
product name | Famotidine EP Impurity D |
CAS | 76824-16-3 |
Product Code | REF-F05005 |
MF | C8H13N5OS2 |
MW | 259.35 |
Purity | 95%+ |

product name | Famotidine EP Impurity D |
CAS | 76824-16-3 |
Product Code | REF-F05005 |
MF | C8H13N5OS2 |
MW | 259.35 |
Purity | 95%+ |
Product nature | Customer customization |
Unit | mg |
Min. Order | 5mg |
Supply Ability | 1000 |
Location | china |
color/storage temp | Store at 2-8℃, dark |
Related Names | Famotidine EP Impurity D,Famotidine EP Impurity DStandard,Famotidine EP Impurity DReference |

TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.

Synthesis of Famotidine EP Impurity D
Complex Impurity Of Famotidine EP Impurity D
Chemical Standards of Famotidine EP Impurity D,
Characterization Of Unknown Impurities of Famotidine EP Impurity D
Structure Profiling of Famotidine EP Impurity D
Identification of Famotidine EP Impurity D
Isolation & Purification of Impurity of Famotidine EP Impurity D
Reference Standards Famotidine EP Impurity D
Research Chemical Famotidine EP Impurity D
Drug substance Famotidine EP Impurity D
Impurity Standards Famotidine EP Impurity D
Reference Standards Famotidine EP Impurity D
Work Standards Famotidine EP Impurity D
Complex Impurity Of Chemical Standards of Famotidine EP Impurity D
Hot Tags:Famotidine EP Impurity D, China, suppliers, manufacturers, factory, customized, price, price list, in stock。
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China