If you have any question, please feel free to email us. We will touch with you as soon as possible.
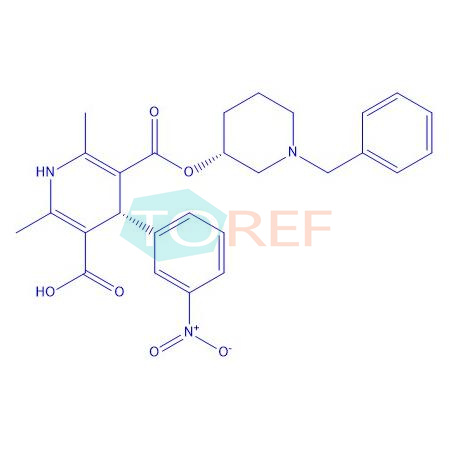
product name | Benidipine Impurity 43 |
CAS | N/A |
Product Code | REF-B19048 |
MF | C27H29N3O6 |
MW | 491.54 |
Purity | 95.63% |

product name | Benidipine Impurity 43 |
CAS | N/A |
Product Code | REF-B19048 |
MF | C27H29N3O6 |
MW | 491.54 |
Purity | 95.63% |
Product nature | Customer customization |
Unit | mg |
Min. Order | 5mg |
Supply Ability | 1000 |
Location | china |
color/storage temp | Store at -20℃ |
Related Names | Benidipine Impurity 43,Benidipine Impurity 43 Standard,Benidipine Impurity 43 Reference |

TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.

Synthesis of Benidipine Impurity 43
Complex Impurity Of Benidipine Impurity 43
Chemical Standards of Benidipine Impurity 43,
Characterization Of Unknown Impurities of Benidipine Impurity 43
Structure Profiling of Benidipine Impurity 43
Identification of Benidipine Impurity 43
Isolation & Purification of Impurity of Benidipine Impurity 43
Reference Standards Benidipine Impurity 43
Research Chemical Benidipine Impurity 43
Drug substance Benidipine Impurity 43
Impurity Standards Benidipine Impurity 43
Reference Standards Benidipine Impurity 43
Work Standards Benidipine Impurity 43
Complex Impurity Of Chemical Standards of Benidipine Impurity 43
Hot Tags:Benidipine Impurity 43, China, suppliers, manufacturers, factory, customized, price, price list, in stock。
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China