If you have any question, please feel free to email us. We will touch with you as soon as possible.
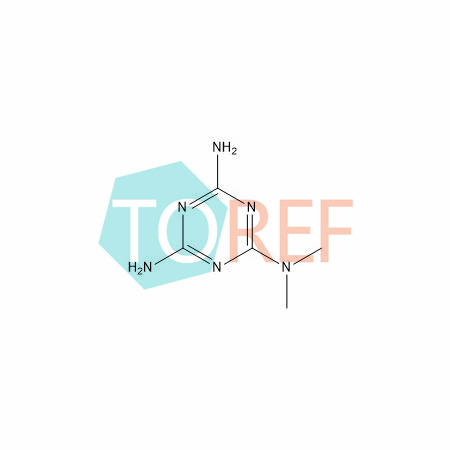
product name | Metformin EP Impurity C |
CAS | 1985-46-2 |
Product Code | REF-M17005 |
MF | C5H10N6 |
MW | 154.18 |
Purity | 98.11% |

product name | Metformin EP Impurity C |
CAS | 1985-46-2 |
Product Code | REF-M17005 |
MF | C5H10N6 |
MW | 154.18 |
Purity | 98.11% |
Product nature | Customer customization |
Unit | mg |
Min. Order | 5mg |
Supply Ability | 1000 |
Location | china |
color/storage temp | Store at 2-8℃ |
Related Names | Metformin EP Impurity C,Metformin EP Impurity CStandard,Metformin EP Impurity CReference |

TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.

Synthesis of Metformin EP Impurity C
Complex Impurity Of Metformin EP Impurity C
Chemical Standards of Metformin EP Impurity C,
Characterization Of Unknown Impurities of Metformin EP Impurity C
Structure Profiling of Metformin EP Impurity C
Identification of Metformin EP Impurity C
Isolation & Purification of Impurity of Metformin EP Impurity C
Reference Standards Metformin EP Impurity C
Research Chemical Metformin EP Impurity C
Drug substance Metformin EP Impurity C
Impurity Standards Metformin EP Impurity C
Reference Standards Metformin EP Impurity C
Work Standards Metformin EP Impurity C
Complex Impurity Of Chemical Standards of Metformin EP Impurity C
Hot Tags:Metformin EP Impurity C, China, suppliers, manufacturers, factory, customized, price, price list, in stock。
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China