
If you have any question, please feel free to email us. We will touch with you as soon as possible.
Korsuva receives FDA approval for the treatment of moderate to severe itching in patients with chronic kidney disease
Today, Cara Therapeutics and Vifor Pharma jointly announced that the US FDA has approved the marketing of Korsuva (difelikefalin) for the treatment of moderate to severe itching in adult patients with chronic kidney disease (CKD) undergoing dialysis. Korsuva is a "first-in-class" KOR agonist that targets the peripheral nervous system. The press release states that this is the first FDA-approved therapy to treat this patient population.
This approval is based on the results of two pivotal Phase 3 clinical trials and supporting data obtained from 32 clinical studies. In two pivotal Phase 3 clinical trials, Korsuva reached the primary endpoint of the trial. Compared with placebo, more patients treated with Korsuva improved the WI-NRS score for pruritus by more than 3 points.
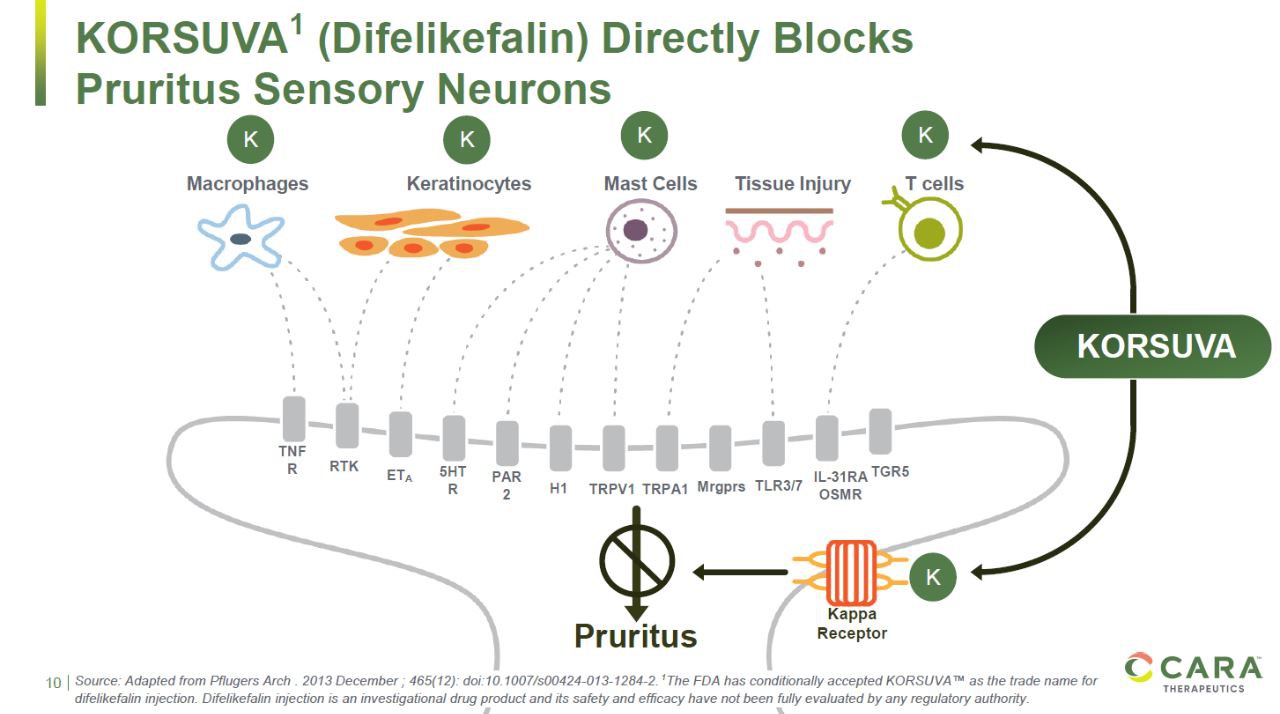
Korsuva is a "first-in-class" KOR agonist, which directly inhibits the activity of peripheral neurons that produce itching, so it can be used to relieve itching due to many reasons. It is currently being used in clinical trials to treat itching caused by a variety of other diseases.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China