
If you have any question, please feel free to email us. We will touch with you as soon as possible.
On August 3, 2021, Genentech, a subsidiary of Roche, announced that the US FDA has granted its heavyweight PD-L1 inhibitor Tecentriq (atezolizumab) Supplementary Biological Products License Application (sBLA) priority review status as Adjuvant treatment for patients with non-small cell lung cancer (NSCLC) after surgery and platinum-containing chemotherapy. The press release pointed out that this is the first tumor immunotherapy that has achieved clinically significant positive results in a Phase 3 clinical trial of adjuvant treatment of early lung cancer.
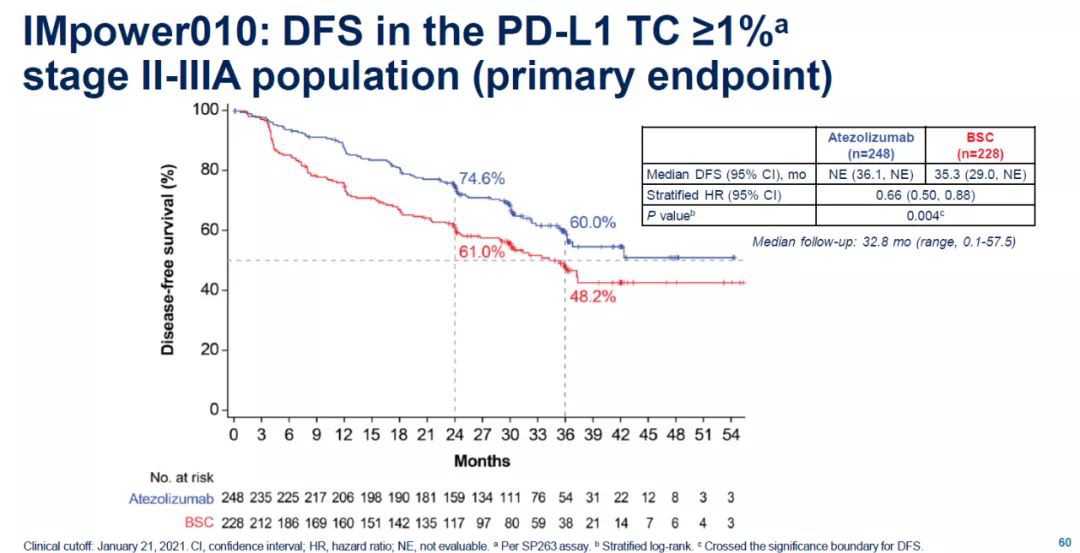
This application is based on the positive interim analysis results obtained from the global Phase 3 IMpower010 clinical trial. The trial enrolled a total of 1005 patients, aiming to evaluate the best standard treatment ( BSC) compared to Tecentriq's efficacy and safety.
The test results showed that compared with BSC, Tecentriq can reduce the risk of disease recurrence by 34% (HR=0.66, 95% CI: 0.50-0.88). When the median follow-up time was 32.8 months, the median disease-free survival (DFS) of the Tecentriq group had not been reached, and the DFS of the BSC group was 35.3 months. The safety data obtained in the test is consistent with the known safety characteristics of the drug, and no new safety signals have been found.
Non-small cell lung cancer accounts for about 80-85% of all lung cancers, and about half of early-stage patients will still experience cancer recurrence after surgery. Tecentriq is a monoclonal antibody that can target the PD-L1 protein expressed on tumor cells and tumor infiltrating immune cells to block its interaction with PD-1 and B7.1 receptors, thereby reactivating immunity T cells to kill tumor cells.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China