
If you have any question, please feel free to email us. We will touch with you as soon as possible.
Recently, Sol-Gel Technologies announced that the US FDA has approved its innovative combination therapy Twyneo cream for the treatment of acne vulgaris (acne vulgaris) in adults and children aged 9 years and older. Twyneo is a fixed-dose compound topical cream containing 0.1% tretinoin and 3% benzoyl peroxide. Clinical trials have proved that it has significant efficacy and good safety characteristics. The press release pointed out that this is also the first fixed-dose combination of these two drugs approved by the FDA.
Acne vulgaris is a common multifactorial skin disease that most commonly occurs in children and adolescents, affecting 80%-85% of adolescents, but it may also occur in adults. Skin lesions appear in areas where a large number of oil glands in the patient’s body, such as the face, chest, neck, and back, are inflammatory (papules, pustules, nodules) or non-inflammatory (acne) lesions. Acne can have a profound impact on the quality of life of patients. In addition to the major risk of permanent facial scars, it may also cause psychological stress, social fear, and decreased self-esteem.
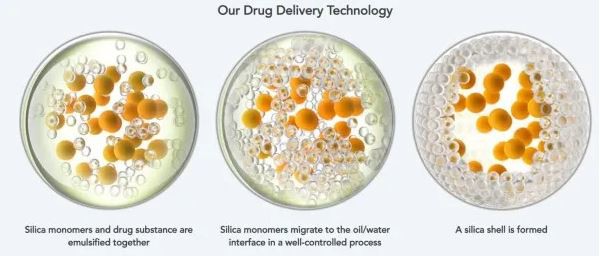
Tretinoin and benzoyl peroxide are widely used in the treatment of acne vulgaris, respectively. If the effects of the two drugs can be used at the same time, the curative effect on patients will be stronger. However, because benzoyl peroxide can cause the degradation of tretinoin molecules, its combination therapy may reduce the efficacy of tretinoin. In order to solve this problem, Twyneo used Sol-Gel's microencapsulation technology to encapsulate tretinoin and benzoyl peroxide in silica microcapsules. This structure can stabilize tretinoin and benzoyl peroxide. It is degraded by benzoyl peroxide and can slowly release the respective active pharmaceutical ingredients over time, resulting in good efficacy and safety characteristics.
The approval is based on the positive results of two randomized, double-blind, excipient-controlled, multi-center Phase 3 clinical trials. Test data show that Twyneo has significant efficacy and well tolerated characteristics in patients with facial acne vulgaris 9 years and older.
"The US FDA's approval of Twyneo demonstrates our ability to provide innovative drugs to the market. Based on the observed clinical data, we believe that Twyneo has the potential to change the treatment landscape for tens of millions of acne patients. We are expected to provide the drug to patients soon I am very excited," said Dr. Alon Seri-Levy, co-founder and CEO of Sol-Gel.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China