
If you have any question, please feel free to email us. We will touch with you as soon as possible.
Recently, Cullinan Oncology announced that the FDA has approved Cullinan Oncology's CLN-619 New Drug Clinical Research Application (IND). CLN-619 is a monoclonal antibody that activates natural killer cells (NK) and T cells through the MICA/B-NKG2D axis, and then exerts anti-tumor activity. It has therapeutic potential for solid tumors and hematological tumors. CLN-619 will also be the first MICA antibody drug to enter human clinical trials.
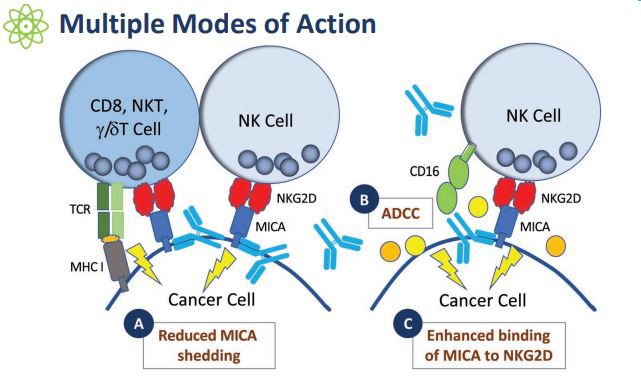
CLN-619 is a humanized IgG1 monoclonal antibody that can bind to MICA/B expressed on a variety of cancer cells. MICA/B is the innate immunity and adaptation of NK cells, CD8+ T cells, and γ/δ T cells. The NKG2D receptor on the surface of sexual immune cells induces a ligand for stress. In order to avoid being lysed by these immune cells, tumor cells will actively shed MICA/B protein on their surface. CLN-619 enhances anti-tumor activity through multiple mechanisms of action, including preventing the proteolytic release of MICA/B from tumor cells, antibody-dependent cell-mediated cytotoxicity (ADCC), enhancing the binding and mitigation of MICA/B and NKG2D Inhibition of MICA/B shedding.
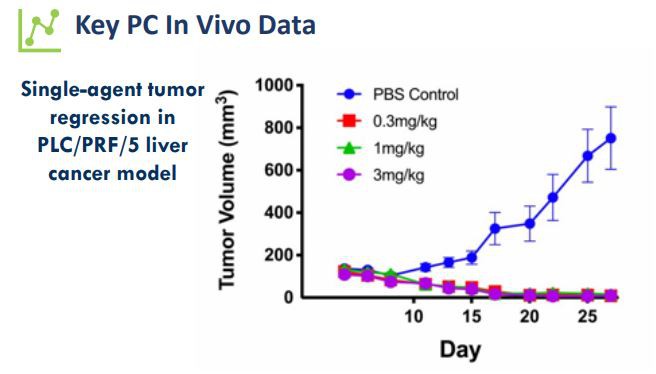
In preclinical studies, animal model data of CLN-619 monotherapy showed significant tumor growth inhibitory effects and a reduction in serum soluble MICA levels.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China