
If you have any question, please feel free to email us. We will touch with you as soon as possible.
Recently, Alkermes announced that the US FDA has approved its new drug
Lybalvi (formerly ALKS 3831) for the treatment of adult schizophrenia and
bipolar I disorder. Lybalvi is an atypical antipsychotic taken orally once a
day. It is a compound tablet composed of olanzapine and samidorphan. It can
maintain the efficacy of olanzapine while reducing its metabolic abnormal side
effects that increase patient weight. .
Olanzapine is a psychiatric drug that has been marketed, but one of its main side effects is that it will significantly increase the weight of patients, thereby increasing their risk of other metabolic diseases. Samidorphan is an opioid receptor antagonist that reduces the weight gain caused by olanzapine by affecting the brain's reward system.
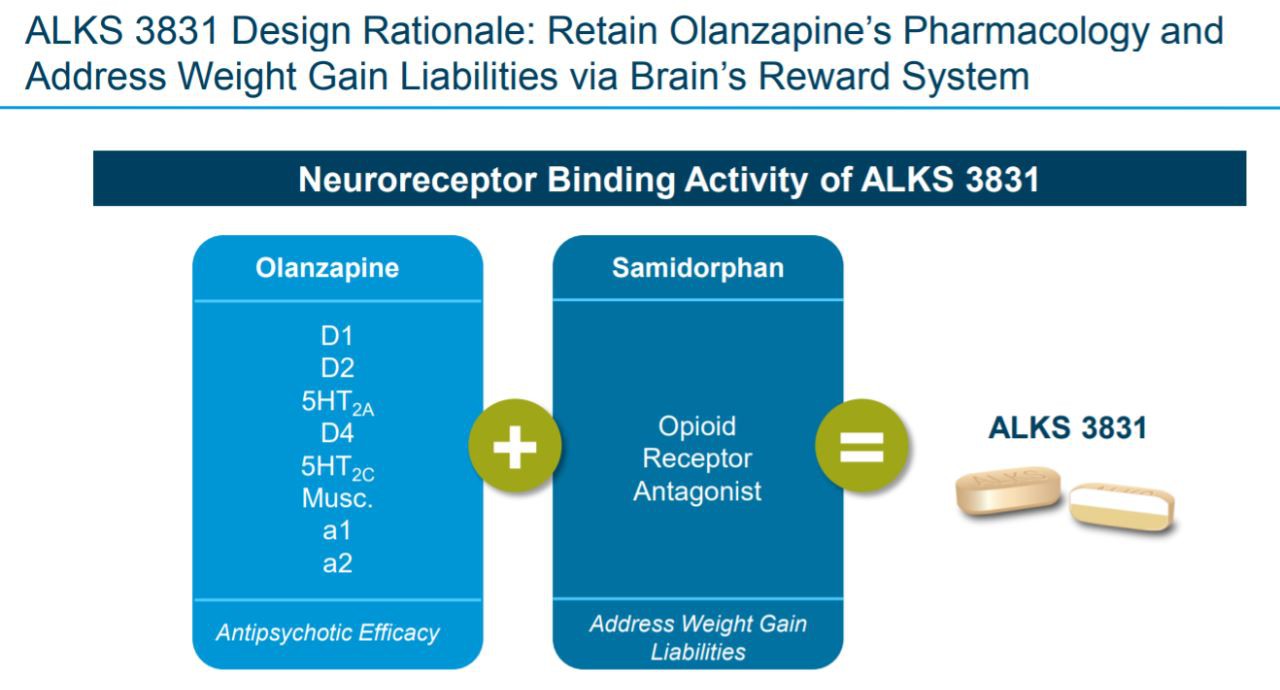
▲The role of Lybalvi (picture source: Alkermes official website)
In the clinical development project of the drug ENLIGHTEN, Lybalvi proved its good effectiveness, safety and tolerability as an antipsychotic. In the multicenter, randomized, double-blind, placebo-controlled Phase 3 clinical trial ENLIGHTEN-2, a total of 561 patients with stable schizophrenia were enrolled. The 6-month treatment results showed that compared with the control group, the average percentage of weight gain of the test group patients showed a statistically significant decrease (p=0.003).
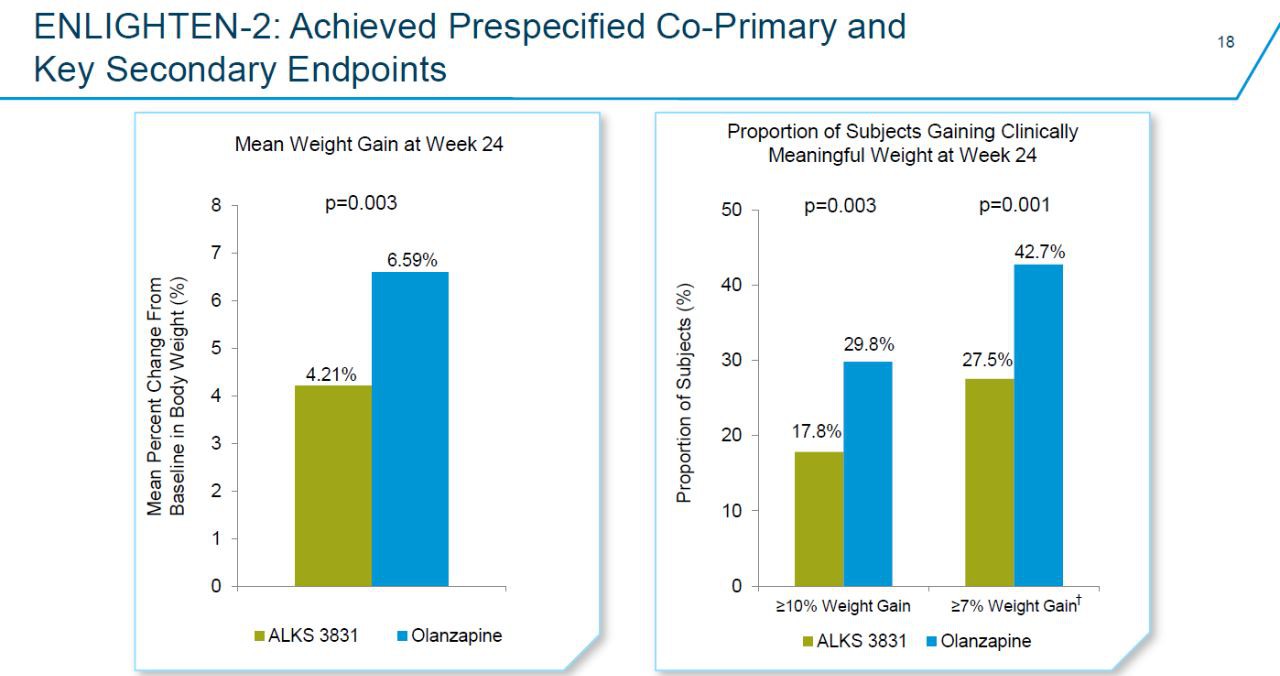
▲Lybalvi significantly reduced the weight gain of patients in the ENLIGHTEN-2 clinical trial (Image source: Alkermes official website)
Bipolar disorder is a disease that can cause severe fluctuations in mood, vitality, and ability to perform daily tasks. Patients may experience extreme mania and depression. They usually need to receive medication, psychotherapy, supportive care, and non-drug physical therapy to control their condition. There is a clinical lack of drugs that can simultaneously control the symptoms of mania and depression in patients. Bipolar I disorder is characterized by the occurrence of at least one manic episode, with or without a major depressive episode.
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China