
If you have any question, please feel free to email us. We will touch with you as soon as possible.
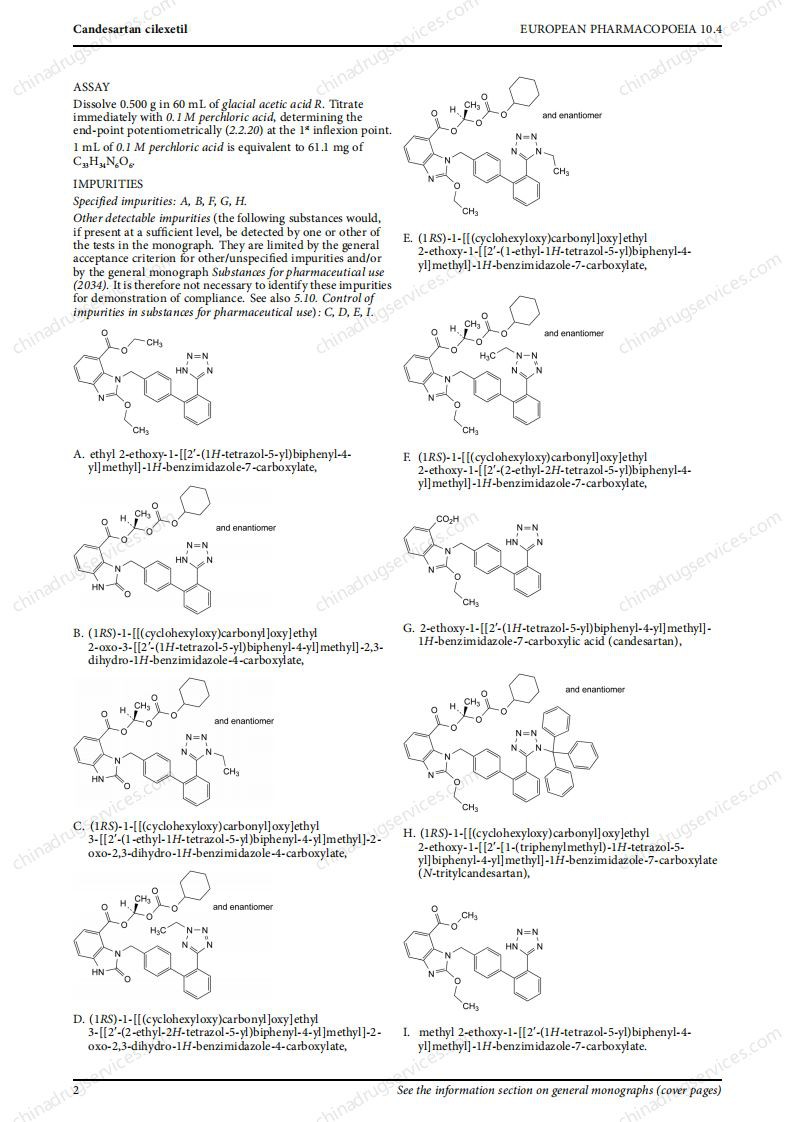
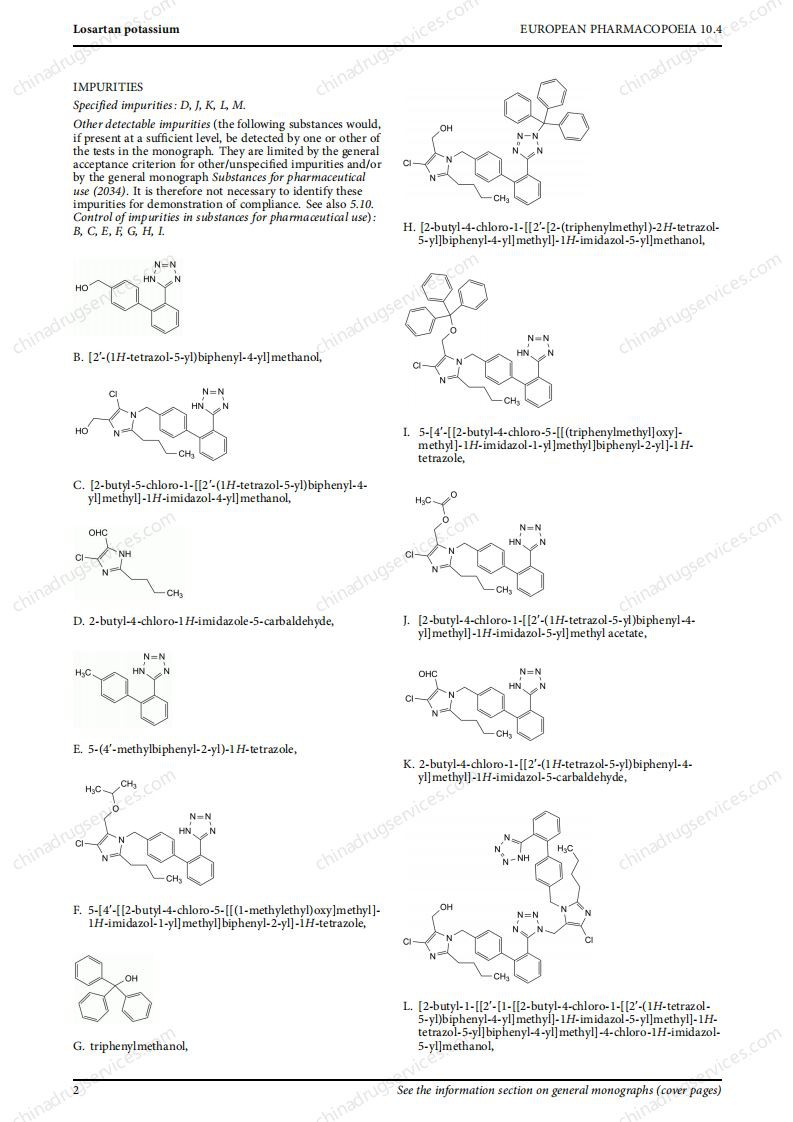
Recently, EDQM revised 5 monographs on sartan drugs with tetrazolium ring, namely valsartan (2423), losartan (2232), irbesartan (2465), candesartan Ester (2573) and Olmesartan (2600) to align them with the latest regulatory recommendations. The revision involves the revision of the "production" part and the deletion of the N-nitrosamine test part.
These revised monographs were not published on Pharmauropa for comment because the revisions made are in line with the CHMP’s recommendations. In addition, to ensure that the implementation date is as consistent as possible with the regulatory decision, the European Pharmacopoeia Commission has decided to publish these monographs in accordance with the rapid revision procedure. Therefore, the implementation date of these 5 revised monographs is set to April 1, 2021.
PDF versions of these monographs can be downloaded from the EDQM website. The revised monograph has been added to the online and downloadable versions of the 10.4 Supplement and 10.5 Supplement. They will be included in the printed version of the 10.6 Supplement.
Sartan drug quality standards:









Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China