If you have any question, please feel free to email us. We will touch with you as soon as possible.
It is commonly used for the diagnosis or treatment of arthritis, joint or tendon problems, skin problems. For R&D purposes only.
* Please be kindly noted products are supplied for RLD supplies/ Reference listed drugs/ Comparator Drug Clinical trials. We do not sell to patients.
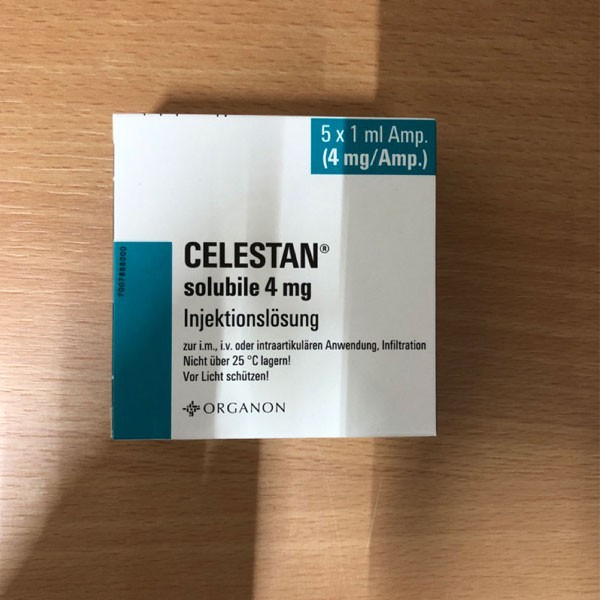
| Generic name | Betamethasone Sodium Phosphate Injection |
| Trade name | Celestone; Celestan; Celesto |
| Specification | 4mg/ml |
| Main ingredients | Betamethasone Sodium Phosphate |
| Dosage Form | Injection |
| MA Holder | MSD Belgium BVBA/SPRL/MSD Sharp & Dohme GmbH/Merck Sharp & Dohme BV/MSD Polska Sp. z o.o/Organon Healthcare GmbH/ Organon Belgium/Organon Polska Sp. z o.o |
| Category | Reference Listed Drugs (RLDs) |
Indications:
It is commonly used for the diagnosis or treatment of arthritis, joint or tendon problems, skin problems.
For more details, pls contact Janney:
Email: tosun-global@upharm.cn
Wechat/Whatsapp:(+86)18922120575
Hot Tags: betamethasone sodium phosphate injection reference listed drug, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Esomeprazole Magnesium Enteric Coated Capsules, Argatroban Injection, Bimatoprost Ophthalmic, Reference Listed Drugs, AdapaleneGel, Phenytoin Sodium Tablets
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China