If you have any question, please feel free to email us. We will touch with you as soon as possible.
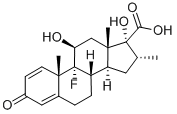
CAS:37927-01-8
MF:C21H27FO5
Melting point:274-2770C
Boiling point566.9±50.0℃(Predicted)
Density 1.35±0.1 g/cm3(Predicted)
| product details: |
| CAS: | 37927-01-8 |
| MF: | C21H27FO5 |
| Melting point: | 274-2770C |
| Boiling point | 566.9±50.0℃(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO(Sparingly),Ethanol (S ghtly),Methanol (Slightly) |
| pka | 3.58±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Molecular Weight | 378.4 g/mo |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Fxact Mass | 378.18425212 g/mo |
| Monoisotopic Mass | 378.18425212 g/mo |
| Topological Polar Surface Area | 94.8A |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 789 |
| sotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
Synonyms
(-)-Dexamethasone Acid
37927-01-8
Dexamethasone acid
Betamethasone acid, (-)-
BP8U81ZN41
UNII-BP8U81ZN41
9-Fluoro-11,17-dihydroxy-16a-methyl-3-oxoandrosta-1,4-diene-17-carboxylic Acid
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthrene-17-carboxylic acid
Dexamethasone sodium phosphate impurity G [EP]
Dexamethasone Sodium phosphate Impurity G ((-)-Dexamethasone Acid)
About TOSUN
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.
For more details, pls contact Janney:
Email: tonghuiyy218@upharm.cn
Wechat/Whatsapp:(+86)18922120635
Hot Tags: dexamethasone sodium phosphate ep impurity g, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Lercanidipine Hydrochloride API, Acarbose Tablets, Clindamycin B, Adapalene Impurity, Oseltamivir Phosphate API, Itraconazole Hydrochloride API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China