If you have any question, please feel free to email us. We will touch with you as soon as possible.
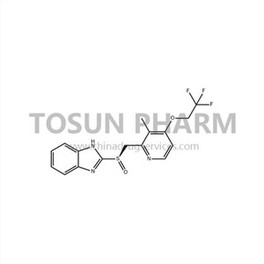
CAS:213476-12-1
Source:India/Europe
Qualifications:USDMF/-/-/-/-
| Name | Dexlansoprazole |
|---|---|
| Chinese name | 右旋兰索拉唑 |
| Cas Number | 213476-12-1 |
| Source | India/Europe |
| Qualifications | USDMF/-/-/-/- |
D-lansoprazole was developed by Takeda and was first listed in the United States in 2009. It has a significant effect on peptic ulcer and gastroesophageal reflux disease. Dexlansoprazole is the enantiomer of the proton pump inhibitor lansoprazole. Compared with lansoprazole after oral administration, its plasma clearance rate is lower and its bioavailability is better. Dexlansoprazole is an oral controlled-release preparation marketed abroad, which is called a biphasic release dosage form. After oral administration, it can reach a second peak in plasma and maintain a longer effective concentration than lansoprazole in plasma. In addition, the binding rate of dextrorotatory plasma protein is greater and the metabolism is slower, which is also the reason for maintaining the effective concentration for a longer time. Post-marketing clinical trials have proved that when taken with clopidogrel at the same time, its active ingredients and inhibit platelet aggregation are less than esomeprazole.
Hot Tags: dexlansoprazole api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Apremilast API, Dapoxetine API, Pregabalin API, Bivalrudine API, Lurasidone Hydrochloride API, Valganciclovir Hydrochloride API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China