If you have any question, please feel free to email us. We will touch with you as soon as possible.
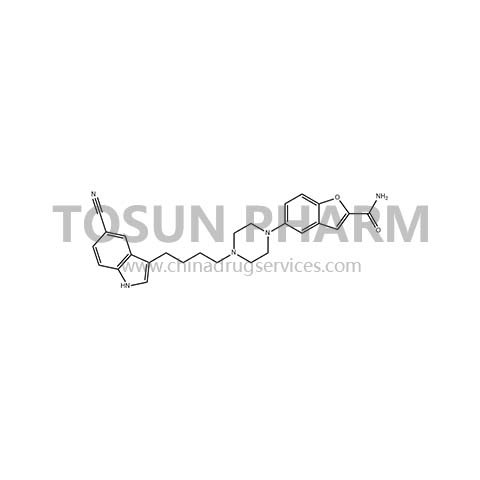
CAS:163521-08-2
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Vilazodone hydrochloride |
|---|---|
| Chinese name | 盐酸维拉佐酮 |
| Cas Number | 163521-08-2 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Verazolone hydrochloride was developed by Trovis Pharmaceuticals LLC of the United States and was approved by the US FDA in 2011 for the treatment of adult major depression. It is the first drug among the new indole alkylamine antidepressants and the first A new type of antidepressant that was screened using pharmacogenomics. Its dual mechanism of action: selective serotonin reuptake inhibitor and 5-HT1A receptor partial agonist, the onset time is significantly better than the existing SSRIs antidepressant drugs, thereby greatly increasing the patient's compliance. Verazolone hydrochloride not only has the mechanism of action of the selective serotonin reuptake inhibitor (SSRI) antidepressant drugs Paroxetine, Prozac and Zoloft, but also has the earliest antidepressant drug buspirone hydrochloride Mechanism. Because it is a new type of antidepressant, vilazodone hydrochloride has a unique mechanism of action, rapid and clear clinical efficacy, and has the advantage of not affecting sexual function like many antidepressants. It has a better prospect than traditional SSRI antidepressants. According to market forecasts, the annual sales of vilazodone hydrochloride are expected to exceed 1 billion US dollars.
Hot Tags: vilazodone hydrochloride api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Ticagrelor API, Ulipristal Acetate API, Tetrabenazine API, Ranolazine API, Active Pharmaceutical Ingredient, Lifitegrast API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China