If you have any question, please feel free to email us. We will touch with you as soon as possible.
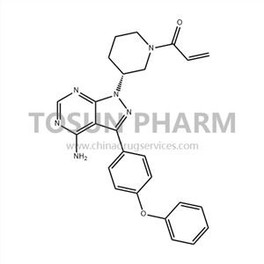
CAS:936563-96-1
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Ibrutinib |
|---|---|
| Chinese name | 伊布替尼 |
| Cas Number | 936563-96-1 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Ibrutinib was jointly developed by Pharmacyclics and Johnson & Johnson. It was approved for marketing by the US Food and Drug Administration in November 2013. It is the first innovative drug belonging to Bruton's tyrosine kinase (BTK) inhibitor. For the treatment of a rare aggressive blood cancer-mantle cell lymphoma (MCL). Ibrutinib irreversibly inhibits BTK by selectively covalently binding to cysteine residues in the active site of the target protein Btk, thereby effectively preventing tumors from migrating from B cells to lymphoid tissues adapted to the tumor growth environment. Compared with traditional chemotherapy, ibrutinib showed better progression-free survival (PFS), overall survival (OS) and overall response rate (ORR). In February 2020, the National Comprehensive Cancer Network (NCCN) guidelines were updated to upgrade Ibrutinib±rituximab from other recommended options to the first choice for the treatment of relapsed/refractory mantle cell lymphoma (MCL) Program. On April 21, 2020, the U.S. Food and Drug Administration (FDA) approved the expansion of the indications for ibrutinib. From 2014 to 2018, the annual global sales of ibrutinib were 471 million US dollars, 1.165 billion US dollars, 2.146 billion US dollars, 3.145 billion US dollars and 4.445 billion US dollars.
Hot Tags: ibrutinib api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Clofarabine API, Oseltamivir Phosphate API, Lurasidone Hydrochloride API, Ranolazine API, Dimethyl Fumarate API, Ticagrelor API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China