If you have any question, please feel free to email us. We will touch with you as soon as possible.
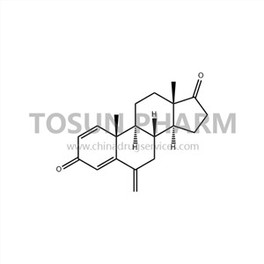
CAS:107868-30-4
Source:India
Qualifications:USDMF/CEP/-/-/-
| Name | Exemestane |
|---|---|
| Chinese name | 依西美坦 |
| Cas Number | 107868-30-4 |
| Source | India |
| Qualifications | USDMF/CEP/-/-/- |
Exemestane tablets were developed and produced by Pfizer. They were approved by the FDA in 1999 and entered the Chinese market on December 17, 2008. Exemestane is a new third-generation steroidal irreversible aromatase inhibitor with low side effects and convenient use. It can irreversibly bind to aromatase to inhibit the biosynthesis of estrogen in the body, thereby reducing circulation and tumor copy The level of estrogen in the site can inhibit the growth of breast cancer, and it has obvious effects on estrogen-dependent tumors such as endometrial cancer. Because exemestane and non-steroidal aromatase inhibitors have different enzyme sites, they have no cross-resistance to each other, and this product may still be effective for patients who use other aromatase inhibitors ineffective. Exemestane can effectively and selectively treat postmenopausal hormone-dependent breast cancer. Because it is well tolerated and has no obvious side effects, exemestane will gradually become the first-line treatment for advanced breast cancer. Compared with megestrol acetate, exemestane is better in terms of effectiveness, safety and side effects, especially in shrinking tumors, prolonging survival and stable disease; compared with similar drugs formestane Compared with exemestane, it can be taken orally and is convenient to use. The sales volume of Exemestane continued to grow after entering the Chinese market. In 2019, it exceeded 300 million yuan to 308 million yuan, a year-on-year increase of 9.61%.
Hot Tags: exemestane api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Lifitegrast API, Adapalene API, Canagliflozin API, Salmeterol Xinafoate API, Vinpocetine API, Lurasidone Hydrochloride API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China