If you have any question, please feel free to email us. We will touch with you as soon as possible.
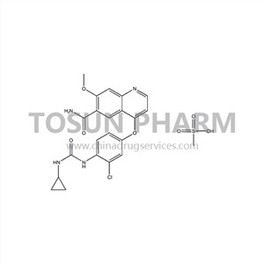
CAS:857890-39-2
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Lenvatinib mesylate |
|---|---|
| Chinese name | 甲磺酸乐伐替尼/仑伐替尼 |
| Cas Number | 857890-39-2 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Lovatinib mesylate was developed by Eisai Japan. It was first granted an orphan drug treatment for thyroid cancer in Japan in August 2012, and was officially approved for marketing in the United States and the European Union in 2015. This product is an oral multi-target kinase inhibitor. The currently approved indications include advanced thyroid cancer, kidney cancer, and hepatocellular carcinoma. It is another ten years after the market for the targeted drug sorafenib for the treatment of liver cancer. New drugs for targeted therapy of liver cancer. It was approved for listing in China in 2018, and was listed as the first-line treatment for non-resectable advanced liver cancer by China's most authoritative cancer diagnosis and treatment guide CSCO liver cancer guide (2018 edition). Lovatinib is a multi-target drug. The main targets of Lovatinib include vascular endothelial growth factor receptor VEGFR1-3, fibroblast growth factor receptor FGFR1-4, platelet-derived growth factor receptor PDGFR-α , CKit, Ret, etc., work by preventing tumors from forming new blood vessels that need to grow. They are the only systemic treatment drugs for liver cancer that can rival or even surpass sorafenib in the past decade.
Hot Tags: lenvatinib mesylate api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Pleasant API, Ulipristal Acetate API, Deoxycholic Acid API, Tippyridine Hydrochloride API, Escitalopram Oxalate API, Teriflunomide API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China