If you have any question, please feel free to email us. We will touch with you as soon as possible.
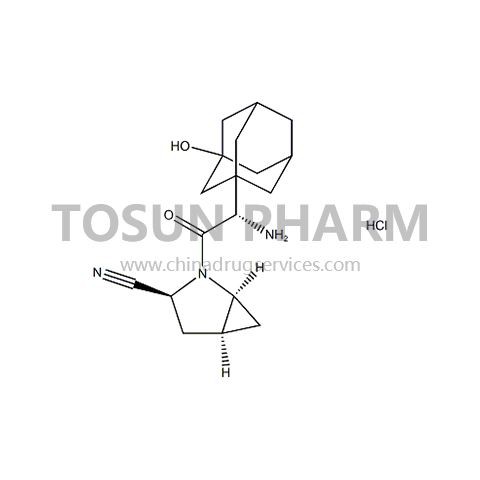
CAS:709031-78-7
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Saxagliptin hydrochloride |
|---|---|
| Chinese name | 盐酸沙格列汀 |
| Cas Number | 709031-78-7 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Saxagliptin is a new dipeptidyl peptidase-IV inhibitor jointly developed by Bristol-Myers Squibb and AstraZeneca. It is the first hypoglycemic based on the mechanism of incretin to be jointly developed by two global pharmaceutical companies. Drugs. In March 2010, the US FDA approved saxagliptin for the treatment of hyperglycemia in adults with type 2 diabetes. In May 2011, it was officially approved by CFDA for listing in China. Saxagliptin can increase endogenous glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-releasing polypeptide (GIP) levels by selectively inhibiting DPP-4, thereby regulating blood sugar. Its hypoglycemic effect is significant, long-lasting and stable, safer than traditional hypoglycemic drugs, and less adverse reactions. It is the first-line clinical medication for hypoglycemic at home and abroad. Compared with existing oral hypoglycemic drugs, saxagliptin only needs to be taken once a day and can be taken at any time of the day, which improves the patient's treatment compliance. Compared with liraglutide, the important advantage of saxagliptin is safety. Patients in the study did not have hypoglycemia or had only a small incidence of hypoglycemia.
Hot Tags: saxagliptin hydrochloride api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Brinzolamide API, Aprepitant Aprepitant API, Pazopanib Hydrochloride Pazopanib API, Lercanidipine Hydrochloride API, Ulipristal Acetate API, Perindopril Tert butylamine Salt API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China