If you have any question, please feel free to email us. We will touch with you as soon as possible.
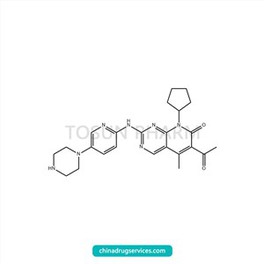
CAS:571190-30-2
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Pabxilib |
|---|---|
| Chinese name | 帕布昔利布 |
| Cas Number | 571190-30-2 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
As the world's first cyclin-dependent kinase (CDK) 4/6 inhibitor, Pabuccilib has become the only breakthrough innovative treatment for advanced breast cancer in China in the past 10 years. In 2013, the FDA approved Pabuccilib as a breakthrough new drug for the treatment of advanced breast cancer. In February 2015, Pabuccilib was approved by the FDA for accelerated postmenopausal breast cancer combined with letrozole (AI drugs) for the treatment of HR+/HER2-, and advanced postmenopausal breast cancer that has not been treated with endocrine therapy for metastases. In February 2016, Pabuccilib was approved by the FDA in combination with Fulvestrant for HR+/HER2-, advanced endocrine therapy, or metastatic breast cancer.
Hot Tags: pabxilib api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Bemeprost API, Lifitegrast API, Oseltamivir Phosphate API, Paliperidone API, Deoxycholic Acid API, Ticagrelor API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China