If you have any question, please feel free to email us. We will touch with you as soon as possible.
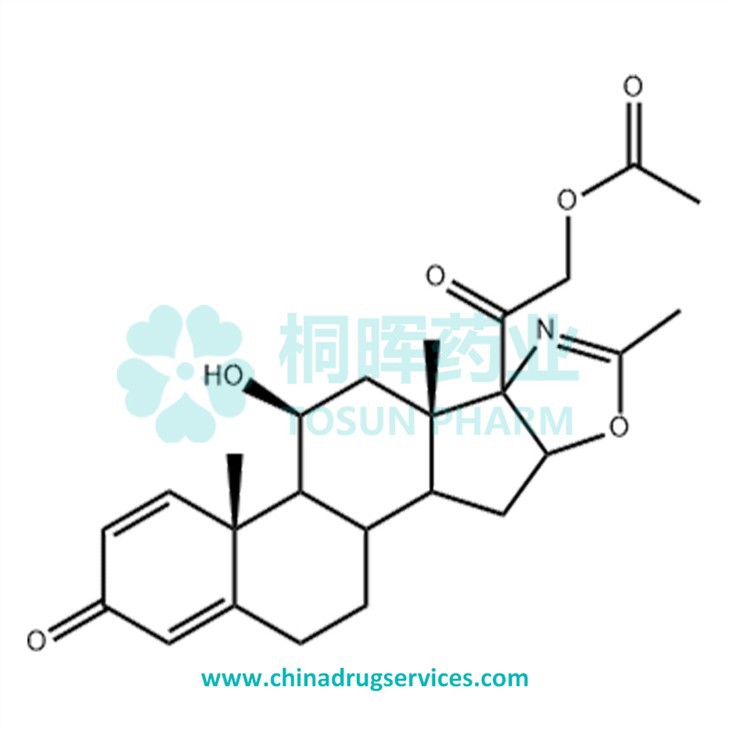
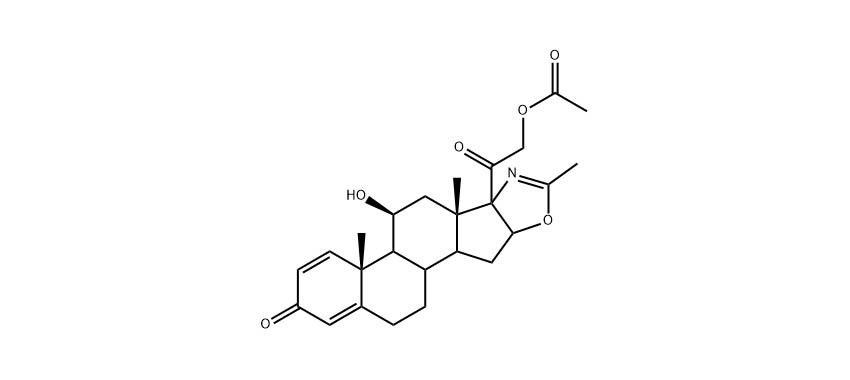
Name: Deflazacort
Chinese Name: 地夫可特
CAS. No.: 14484-47-0
Molecular Formula: C25H31NO6
Molecular Weight: 441.52 g/mol
Source: Europe
Qualifications: USDMF/-/-/-/-
| Name | Deflazacort |
|---|---|
| Chinese Name | 地夫可特 |
| CAS. No. | 14484-47-0 |
| Molecular Formula | C25H31NO6 |
| Molecular Weight | 441.52 g/mol |
| Synonyms | (5'β)-21-Acetyloxy-11β-hydroxy-2'-methylpregnano[17,16-d]oxazole-1,4-diene-3,20-dione |
| Class | Active Pharmaceutical Ingredients (API) & Intermediates, API |
| Industry | Pharmaceutical |
| Appearance | White to Tan Powder |
| Source | Europe |
| Qualifications | USDMF/-/-/-/- |
Deflazacort is a third-generation steroidal glucocorticoid drug developed by Marathon Pharmaceuticals and approved by the US FDA for the treatment of Duchenne muscular dystrophy in February 2017. Deflazacort is rapidly metabolized to active 21-OH deflazacort, which exhibits anti-inflammatory and immunosuppressive effects by acting on glucocorticoid receptors. Compared with other glucocorticoids, the efficacy of deflazacort in the treatment of rheumatoid arthritis and systemic lupus erythematosus is earlier and longer lasting. However, its side effects, especially in promoting the excretion of calcium in the urine and inhibiting the intestinal absorption of calcium, are weaker than those of prednisolone acetate and betamethasone, so the side effects of bone loss are weaker. As the third-generation glucocorticoid, deflazacort has a stable curative effect and few side effects, and its sales volume has been stable since its launch. In 2014, the global sales volume was 93.88 million US dollars, and because it was approved by the US FDA for the treatment of Duchenne muscular dystrophy, It will play a very beneficial role in promoting its sales.
Storage: It should be kept refrigerated between 2°C to 8°C.

Regardless of the type of drug or the shape it takes, all products need to be handled with great care. We can offer you different shipping support from production to delivery, or anywhere in between. You can feel at ease knowing your products will be in good hands.

Guangzhou Tosun Pharmaceutical Ltd. is a high-teched enterprise with modern workshops and advanced production technology, located in Guangzhou, China and with nearly 200 staff members. The construction area is more than 3,000 square meters.
With a global network of procurement, R&D and marketing, it can quickly meet the needs of domestic and foreign pharmaceutical enterprises in products of R&D, production, and marketing sales. Now, about 40% of our products are exported to foreign markets, including Europe, India, Korea, Japan, US, and so on. We have established good cooperation with customers at home and abroad.
Q1: How to know the exact price for this product?
A: Please provide your email address and detailed order information, then we can check and reply to you with the latest and exact price.
Q2: We have more questions, how to contact you?
A: We are a renowned and authentic manufacturer and supplier of Deflazacort in China. At Tosun Pharm, we have expertise in synthetic organic chemistry and pharmaceutical analysis and we provide cost-effective and accurate custom research services to the industry. Do you want a quotation or have a question about Deflazacort? Please Enquire Now! You can send inquiries through the inquiry form below or contact us by Email or Phone/ WhatsApp directly. We normally respond within 12 hours.
Phone/ WhatsApp: +86-189-2212-0635
Email: tonghuiyy218@upharm.cn
Hot Tags: deflazacort api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Ranolazine API, Salmeterol Xinafoate API, Solinasine Succinate API, Perindopril Arginine API, Aprepitant Aprepitant API, Bosentan API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China