If you have any question, please feel free to email us. We will touch with you as soon as possible.
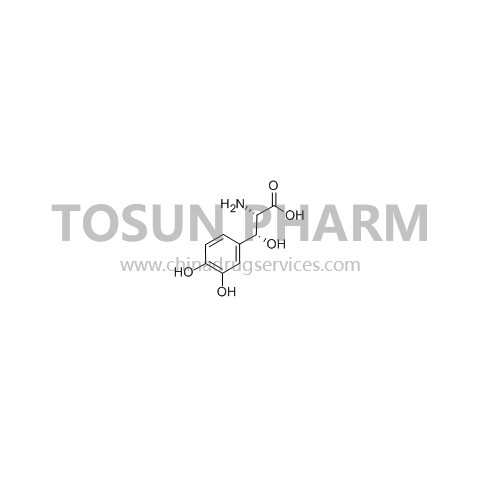
CAS:23651-95-8
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Droxidopa |
|---|---|
| Chinese name | 屈昔多巴 |
| Cas Number | 23651-95-8 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Droxidopa is a known synthetic norepinephrine amino acid precursor and has been authorized as a rare disease drug in the United States. On February 18, 2014, the U.S. FDA approved the accelerated approval of Droxidopa developed by Danish Lingbei. Listed. Droxidopa is the first and only drug approved by the US Food and Drug Administration (FDA) for the treatment of NOH, and it is also the first new treatment option for the symptomatic treatment of NOH in the past two decades. Droxidopa is a known synthetic norepinephrine amino acid precursor, which is directly converted into norepinephrine by the action of dopa decarboxylase (DDC). The clinical research conclusions show that the droxidopa treatment group can reduce the value of orthostatic blood pressure drop, which is significantly better than the control group. During the entire treatment process, the patient did not develop tachycardia or bradycardia symptoms, showing its effectiveness and safety. According to US IMS data in 2017, in the global top 500 best-selling drug market, the anti-Parkinson therapy market was US$2.056 billion, a year-on-year increase of 3.73%. In 2017, the global Northera market sales of Doxydopa reached 245 million U.S. dollars, an increase of 51.24% year-on-year. It became a fast-growing variety in the global anti-PD market in 2017 and a T0P5 variety in the anti-PD market.
Hot Tags: droxidopa api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Lurasidone Hydrochloride API, Ulipristal Acetate API, Clofarabine API, Lifitegrast API, Fulvestrant API, Perindopril Tert butylamine Salt API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China