If you have any question, please feel free to email us. We will touch with you as soon as possible.
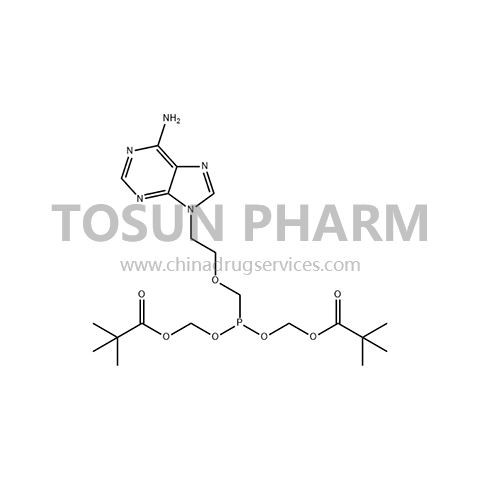
CAS:142340-99-6
Source:Korea
Qualifications:-/-/-/-/-
| Name | Adefovir dipivoxil |
|---|---|
| Chinese name | 阿德福韦酯 |
| Cas Number | 142340-99-6 |
| Source | Korea |
| Qualifications | -/-/-/-/- |
Adefovir dipivoxil is an oral broad-spectrum antiviral drug originally synthesized by Gilead in the United States and approved by the FDA for marketing in the United States in September 2002. It is also another oral anti-HBV drug following lamivudine. The American Association for the Study of Liver Diseases, the Asia-Pacific Society for Liver Research, and China's "Guidelines for the Prevention and Treatment of Chronic Hepatitis B" recommend adefovir dipivoxil as the first-line choice for newly treated patients. Adefovir dipivoxil can inhibit the activity of HBV DNA polymerase, and can penetrate into the hepatitis B virus DNA to inhibit virus replication. It has a strong inhibitory effect on HBV strains resistant to lamivudine and famciclovir. It is clinically used in the treatment of hepatitis. US Patent No. 5,663,159 reported that the oral bioavailability (17.0) of adefovir dipivoxil is more than twice that of adefovir (7.8). The results of clinical trials for the treatment of active hepatitis B complicated by cirrhosis show that, compared with lamivudine alone, the combined application of lamivudine and adefovir dipivoxil can effectively control the hepatitis B virus load of patients. Relieve substantial liver damage, reduce the development of cirrhosis, and have significant clinical effects. The domestic drugs used to treat hepatitis B are divided into interferon and nucleoside drugs. Among them, nucleoside drugs account for about 80% of the market share; in 2019, the sales of adefovir dipivoxil capsule sample hospitals in China's sample hospitals reached 325 million yuan.
Hot Tags: adefovir dipivoxil api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Pregabalin API, Tippyridine Hydrochloride API, Paliperidone API, Fulvestrant API, Ulipristal Acetate API, Tavaborol API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China