If you have any question, please feel free to email us. We will touch with you as soon as possible.
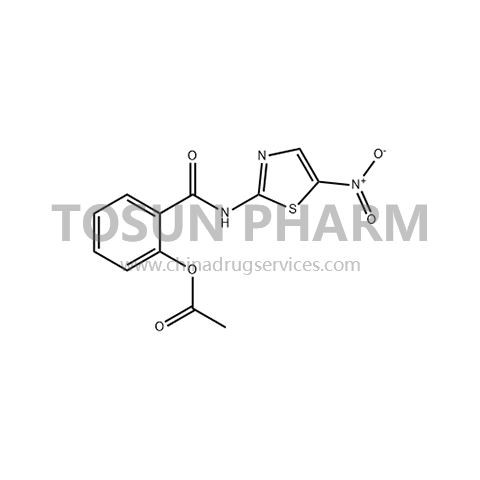
CAS:55981-09-4
Source:Europe
Qualifications:USDMF/-/-/-/-
| Name | Nitazoxanide |
|---|---|
| Chinese name | 硝唑尼特 |
| Cas Number | 55981-09-4 |
| Source | Europe |
| Qualifications | USDMF/-/-/-/- |
Nitrazoxanide was first developed by Romark Laboratories in the United States. It is an amide anti-anaerobic drug with broad-spectrum antiprotozoal, worm and bacterial properties, especially for the treatment of cryptosporidiosis in AIDS patients infection. It is the first and only drug approved by the U.S. Drug and Food Administration (FDA) for the treatment of cryptosporidiosis and the only approved drug for the treatment of aquatic parasitic infections. The mechanism of action of nitazoxanide and anaerobic bacteria is to directly inhibit the pyruvate ferredoxin oxidoreductase reaction. After repeated clinical trials abroad, it has specific effects on children's diarrhea caused by Cryptosporidium and Giardia lamblia. It also has a therapeutic effect on hepatitis B, hepatitis C, and gastrointestinal viral infections such as dysentery and diarrhea. It is known as the treatment of hepatitis B and Important development of hepatitis C. There are some preliminary research results that show that in the treatment of hepatitis B with nitazoxanide, the conversion rate of HbeAg and HBsAg is significantly higher than that of any other approved hepatitis B treatment drugs including interferon.
Hot Tags: nitazoxanide api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Temozolomide API, Fondaparinux API, Lapatinib Ditosylate API, Gabapentin API, Lifitegrast API, Letrozole API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China