If you have any question, please feel free to email us. We will touch with you as soon as possible.
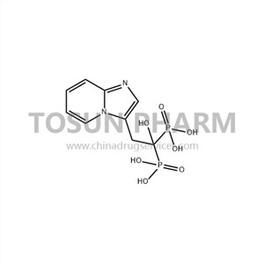
CAS:180064-38-4
Source:Europe
Qualifications:-/-/-/-/-
| Name | Minodronic acid |
|---|---|
| Chinese name | 米诺膦酸 |
| Cas Number | 180064-38-4 |
| Source | Europe |
| Qualifications | -/-/-/-/- |
Minodronic acid is a third-generation nitrogen-containing aromatic heterocyclic bisphosphonate compound. It was developed by Japan Astellas Pharmaceuticals, and jointly developed by Ono Pharmaceuticals. It was approved by the Ministry of Health and Welfare of Japan in January 2009. Acid tablets are marketed for the treatment of osteoporosis and hypercalcemia caused by osteoporosis and malignant tumors. Compared with the third-generation bisphosphonate alendronic acid, minodronic acid has a similar effect, but the dosage is significantly reduced, which improves patient compliance and reduces common gastrointestinal adverse reactions of bisphosphonates; it inhibits bone The absorption activity is 2 times, 10 times and 100 times that of incadronate disodium, alendronate sodium and pamidronate disodium respectively. The research and development of minodronic acid will undoubtedly provide a safer, effective and convenient therapeutic drug for patients with clinical diseases, and it will surely produce good social and economic benefits after it is marketed.
Hot Tags: minodronic acid api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Ticagrelor API, Troxagliptin Succinate API, Lurasidone Hydrochloride API, Lifitegrast API, Edoxaban API, Bivalrudine API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China