If you have any question, please feel free to email us. We will touch with you as soon as possible.
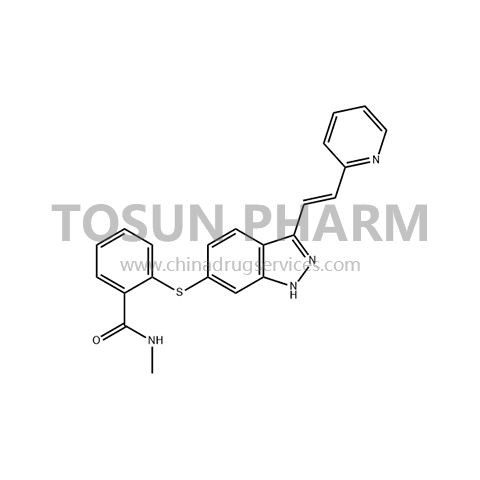
CAS:319460-85-0
Source:India
Qualifications:-/-/-/-/-
| Name | Axitinib |
|---|---|
| Chinese name | 阿西替尼 |
| Cas Number | 319460-85-0 |
| Source | India |
| Qualifications | -/-/-/-/- |
Axitinib is a multi-target tyrosine kinase inhibitor developed by Pfizer. It can inhibit vascular endothelial cell growth factor receptors VEGFR1, VEGFR2, VEGFR3, platelet-derived growth factor receptor and c-KIT, and is used in other system treatments. The ineffective advanced renal cell carcinoma was approved by the FDA on January 27, 2012. Compared with sunitinib, pembrolizumab + axitinib first-line treatment of advanced renal cell carcinoma can significantly improve OS, PFS and objective response rate ORR. In a phase III clinical trial, compared with sorafenib, axitinib significantly prolonged progression-free survival and showed overall good safety for patients with advanced renal cell carcinoma who had previously received treatment. In April 2019, based on the results of the KEYNOTE-426 study, the FDA approved pembrolizumab combined with axitinib for the first-line treatment of advanced renal cell carcinoma. This is also the first approved immune + targeted drug combination. In March 2020, teriprizumab combined with axitinib for the treatment of mucosal melanoma was granted orphan drug designation by the US FDA. In 2018, sales of axitinib were US$276 million.
Hot Tags: axitinib api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Itraconazole Hydrochloride API, Valganciclovir Hydrochloride API, Pabxilib API, Ticagrelor API, Ulipristal Acetate API, Canagliflozin API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China