If you have any question, please feel free to email us. We will touch with you as soon as possible.
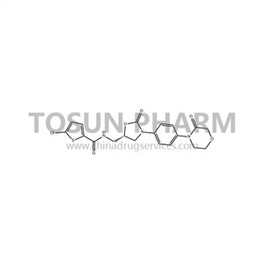
CAS:366789-02-8
Source:India
Qualifications:-/-/-/-/-
| Name | Rivaroxaban |
|---|---|
| Chinese name | 利伐沙班 |
| Cas Number | 366789-02-8 |
| Synonyms | (S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carbox |
| Class | Active Pharmaceutical Ingredients (API) & Intermediates, API |
| Industry | Pharmaceutical |
| Molecular Formula | C19H18ClN3O5S |
| Appearance | Solid |
| Molecular Weight | 435.88 |
| Source | India |
| Qualifications | -/-/-/-/- |
Rivaroxaban is a selective coagulation factor Xa inhibitor. By competitively inhibiting free and bound factor Xa, it interrupts the endogenous and exogenous pathways of the coagulation cascade, inhibits the production of thrombin, and exerts anti-thrombotic effects. effect. The original drug was jointly developed by Bayer and Johnson & Johnson's subsidiary Xi'an Janssen. It was approved by the EMA in September 2008 and the FDA in November 2011. It is a blockbuster product in the anticoagulation market and has been approved by more than 130 countries around the world. Rivaroxaban is the most widely used non-vitamin K antagonist oral anticoagulant (NOAC) in the world. It has been approved for 9 therapeutic indications, and the indications are different in different countries. Compared with other NOACs, rivaroxaban can help a wide range of patient groups to prevent a variety of venous thromboembolism (VTE) and arterial thromboembolism (VAT) diseases. In October 2019, Rivaroxaban was approved by the U.S. FDA for the prevention of VTE in hospitalized patients with acute medical diseases who are at risk of thromboembolic complications but not at high risk of bleeding. In August 2020, the China Medical Products Administration approved the combined administration of rivaroxaban tablets and aspirin for patients with chronic coronary artery disease or peripheral artery disease to reduce the risk of major cardiovascular events.
Storage: It should be kept refrigerated between 2°C to 8°C.

Regardless of the type of drug or the shape it takes, all products need to be handled with great care. We can offer you different shipping support from production to delivery, or anywhere in between. You can feel at ease knowing your products will be in good hands.

Guangzhou Tosun Pharmaceutical Ltd. is a high-teched enterprise with modern workshops and advanced production technology, located in Guangzhou, China and with nearly 200 staff members. The construction area is more than 3,000 square meters.
With a global network of procurement, R&D and marketing, it can quickly meet the needs of domestic and foreign pharmaceutical enterprises in products of R&D, production, and marketing sales. Now, about 40% of our products are exported to foreign markets, including Europe, India, Korea, Japan, US, and so on. We have established good cooperation with customers at home and abroad.
Q1: How to know the exact price for this product?
A: Please provide your email address and detailed order information, then we can check and reply to you with the latest and exact price.
Q2: We have more questions, how to contact you?
A: We are a renowned and authentic manufacturer and supplier of Rivaroxaban in China. At Tosun Pharm, we have expertise in synthetic organic chemistry and pharmaceutical analysis and we provide cost-effective and accurate custom research services to the industry. Do you want a quotation or have a question about Rivaroxaban? Please Enquire Now! You can send inquiries through the inquiry form below or contact us by Email or Phone/ WhatsApp directly. We normally respond within 12 hours.
Phone/ WhatsApp: +86-189-2212-0635
Email: tonghuiyy218@upharm.cn
Hot Tags: rivaroxaban api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Itraconazole Hydrochloride API, Troxagliptin Succinate API, Rasagiline Mesylate API, Solinasine Succinate API, Pleasant API, Dapoxetine API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China