If you have any question, please feel free to email us. We will touch with you as soon as possible.
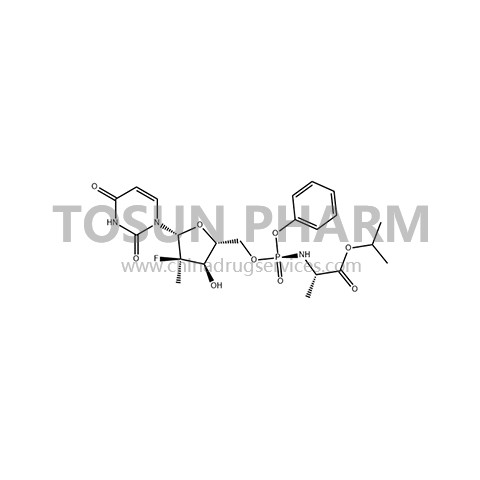
CAS:1190307-88-0
Source:India
Qualifications:-/-/-/-/-
| Name | Sofosbuvir/Sofosbuvir |
|---|---|
| Chinese name | 索磷布韦/索非布韦 |
| Cas Number | 1190307-88-0 |
| Source | India |
| Qualifications | -/-/-/-/- |
Sofosbuvir is the first specific HCV nucleotide analogue NS5B polymerase inhibitor (400 mg) developed by Pharmasset and Gilead. The drug was approved for marketing in the United States in December 2013, and was launched in more than a dozen European countries including Germany in January 2014. It was launched in China in September 2017. Sofosbuvir is effective against all 6 genotypes of HCV. Sofosbuvir only needs to be taken orally once a day, with few drug interactions and relatively few reports of adverse reactions. In 2016, the World Health Organization's hepatitis C guidelines recommended that sofosbuvir and dacatavir should be combined to treat HCV types 1, 3 and 4, and sofosbuvir should be combined with Harvoni to treat genes 1, 4, and 5. And type 6 HCV, combined with ribavirin for the treatment of gene types 2 and 3.
Hot Tags: sofosbuvir/sofosbuvir api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Aprepitant Aprepitant API, Bemeprost API, Valganciclovir Hydrochloride API, Paliperidone API, Gabapentin API, Linagliptin API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China