If you have any question, please feel free to email us. We will touch with you as soon as possible.
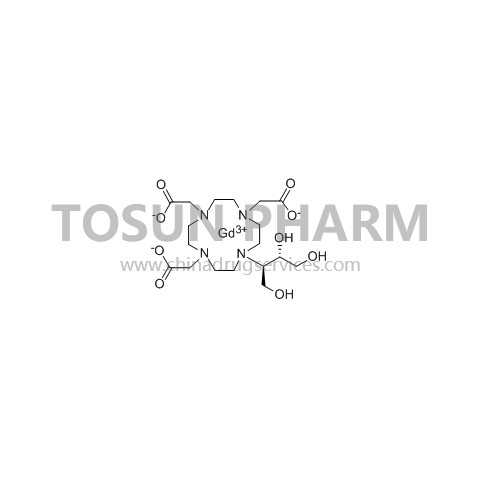
CAS:138071-82-6
Source:Korea
Qualifications:-/-/-/-/-
| Name | Gadobutrol |
|---|---|
| Chinese name | 钆布醇 |
| Cas Number | 138071-82-6 |
| Source | Korea |
| Qualifications | -/-/-/-/- |
Gadobutrol was first approved in Switzerland in February 1998, and it has been approved by more than 100 countries. In 2018, it was approved by NMPA for domestic marketing. Gadobutrol is one of the three low-risk gadolinium-based contrast agents recommended by the European guidelines. It has a non-inferior effect on CNS imaging with the contrast agent gadoterol. It is the only gadolinium-based contrast agent approved by the FDA for neonatal use. In view of its high concentration and high relaxation efficiency, it shows the highest short T1 efficiency per milliliter among all gadolinium-containing contrast agents, which makes it have excellent image quality and has the advantages of a smaller dosage. In April this year, gadobutrol was again approved for use in magnetic resonance imaging examinations for children under 2 years old (including full-term newborns), becoming the first high-concentration, high-relaxation rate, and large-scale indication for children under 2 years old in China Ring magnetic resonance contrast agent. In 2018, the sales of terminal contrast agents in public medical institutions in China was 13.773 billion yuan, a year-on-year increase of 20.21%, and the sales of gadobutrol injection was 32.36 million yuan. The contrast agent market continues to rise, and the market prospects for gadobutrol are promising in the long term.
Hot Tags: gadobutrol api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Pazopanib Hydrochloride Pazopanib API, Perindopril Tert butylamine Salt API, Idebenone API, Deoxycholic Acid API, Active Pharmaceutical Ingredient, Oseltamivir Phosphate API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China