If you have any question, please feel free to email us. We will touch with you as soon as possible.
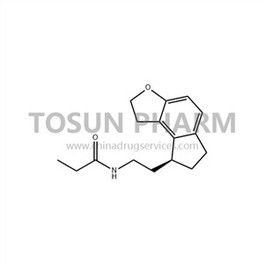
CAS:196597-26-9
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Ramelteon |
|---|---|
| Chinese name | 雷美替胺 |
| Cas Number | 196597-26-9 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Ramelteamide is the first melatonin receptor agonist sedative and hypnotic drug, developed by Takeda, Japan. This product was first launched in the United States on July 22, 2005, and was approved for marketing in Japan in April 2010; available It is used for the treatment of insomnia that is difficult to fall asleep, and it also has a definite effect on chronic and short-term insomnia. This product can selectively stimulate the type 1 and type 2 receptors of melatonin (MT1, MT2), increase slow wave sleep (SWS) and rapid eye movement sleep (REW), thereby reducing insomnia. Ramelteamide is the first non-addictive insomnia treatment drug that is not under special control. This product has significant effects in the treatment of insomnia and difficulty falling asleep. It is safe to use, has a wide therapeutic window, few adverse reactions, and does not have long-term medication. Produce drug dependence.
Hot Tags: ramelteon api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Canagliflozin API, Temozolomide API, Celecoxib API, Fondaparinux API, Bivalrudine API, Adapalene API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China