If you have any question, please feel free to email us. We will touch with you as soon as possible.
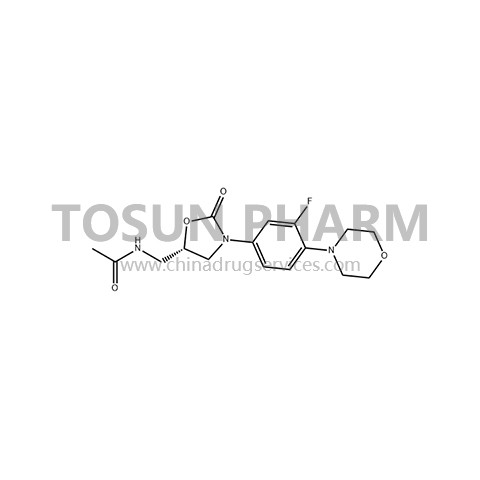
CAS:165800-03-3
Source:India/Taiwan
Qualifications:USDMF/-/-/-/-
| Name | Linezolid |
|---|---|
| Chinese name | 利奈唑胺 |
| Cas Number | 165800-03-3 |
| Source | India/Taiwan |
| Qualifications | USDMF/-/-/-/- |
Linezolid is a synthetic oxazolidinone antibiotic. The original research company is Pfizer. It was first approved by the US FDA in 2000 for the treatment of infections caused by gram-positive (G+) cocci, including methicillin-resistant golden yellow grapes. Suspected or confirmed hospital-acquired pneumonia (HAP), community-acquired (CAP), complicated skin or skin and soft tissue infections (SSTI), and vancomycin-resistant enterococcus (VRE) infections caused by bacteria (MRSA). Not only is its chemical structure different from other antibacterial drugs, but its mechanism of action also has new features. Because linezolid has a unique site and mode of action, it is not easy to cross-resistance with other antibacterial drugs that inhibit protein synthesis, and it is not easy to induce resistance in vitro, making it resistant to MRSA, VRE, and multi-drug resistant Streptococcus pneumoniae (MDRSP) etc. have strong antibacterial activity. Related guidelines suggest that linezolid is the first choice for the treatment of hospital-acquired pneumonia and soft tissue infections in MRSA. In 2019, the domestic market size of linezolid injection was 1.48 billion yuan.
Hot Tags: linezolid api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Canagliflozin API, Lapatinib Ditosylate API, Pleasant API, Solinasine Succinate API, Ranolazine API, Tetrabenazine API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China