If you have any question, please feel free to email us. We will touch with you as soon as possible.
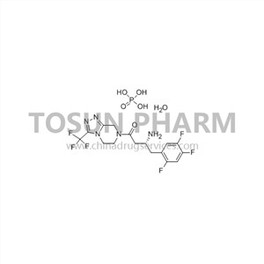
CAS:654671-77-9
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Sitagliptin Phosphate |
|---|---|
| Chinese name | 磷酸西格列汀 |
| Cas Number | 654671-77-9 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Sitagliptin phosphate was approved by the FDA in October 2006 under the trade name Genovi. Sitagliptin phosphate is the first DPP-4 inhibitor approved for the treatment of type 2 diabetes. It can inhibit β cell apoptosis, promote β cell regeneration, increase the number of β cells in patients with type 2 diabetes, and significantly reduce blood sugar in patients. It still has a significant hypoglycemic effect on patients whose sulfonylureas have failed. Sitagliptin phosphate combined with insulin detemir has a significant effect in the treatment of type 2 diabetes, and can better control the blood sugar content, reduce blood sugar fluctuations, reduce the risk of hypoglycemia, and have better safety. It is worthy of further clinical promotion. With the increasing incidence of type 2 diabetes, sitagliptin phosphate has become more and more popular as a new anti-diabetic drug. Sitagliptin phosphate tablets have become the second largest drug in the US oral diabetes drug market.
Hot Tags: sitagliptin phosphate api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Ranolazine API, Salmeterol Xinafoate API, Dapoxetine API, Troxagliptin Succinate API, Edoxaban API, Oseltamivir Phosphate API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China