If you have any question, please feel free to email us. We will touch with you as soon as possible.
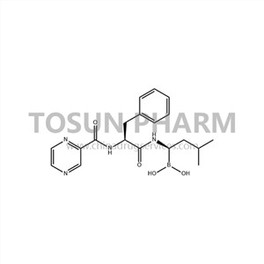
CAS:179324-69-7
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Bortezomib |
|---|---|
| Chinese name | 硼替佐米 |
| Cas Number | 179324-69-7 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
In May 2003, Bortezomib was approved by the U.S. Food and Drug Administration. In 2016, its global sales reached 2.5 billion U.S. dollars. It has been ranked in the TOP10 global anti-tumor brand drug sales list for several consecutive years. It is a veritable blockbuster drug. It was approved to enter the Chinese market in 2005. It is the first approved first-in-class oral drug for multiple myeloma in China and is currently the first-line specific targeted drug for the clinical treatment of multiple myeloma. Bortezomib is a synthetic alanine-based boronic acid derivative, which reversibly inhibits the 26S proteasome activity, causing the degradation of many important proteins in the cell to be blocked, thereby activating the apoptosis pathway and inducing tumor cell apoptosis. Die. Its mechanism of action won the 2004 Nobel Prize in Chemistry, and in 2006 it won the highest honor in the pharmaceutical industry-the Prix Galien Award (Prix Galien), known as "a revolution in tumor treatment and a major advancement in the treatment of multiple myeloma." Clinical studies have confirmed that bortezomib has a definite effect on relapsed and refractory multiple myeloma. The adverse reactions are relatively mild and can be tolerated, especially in relieving pain and discomfort, improving sleep and rest, and getting rid of drugs and medical treatments. The dependence and other aspects have significant clinical significance.
Hot Tags: bortezomib api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Cinacalcet Hydrochloride API, Escitalopram Oxalate API, Adapalene API, Itraconazole Hydrochloride API, Tetrabenazine API, Tippyridine Hydrochloride API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China