If you have any question, please feel free to email us. We will touch with you as soon as possible.
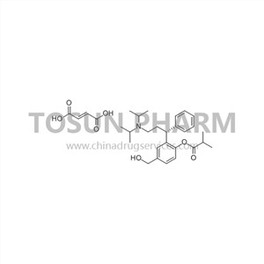
CAS:286930-03-8
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Fesoterodine fumarate |
|---|---|
| Chinese name | 富马酸非索罗定 |
| Cas Number | 286930-03-8 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
The original research company of fesoterodine fumarate sustained-release tablets was Pfizer. It was approved by EMA on April 20, 2007, and was approved by FDA on October 31, 2008. It is a treatment for overactive bladder syndrome. The prodrug, which enters the body, is converted into the active metabolite 5-HMT of a muscarinic receptor antagonist, which can be used for the treatment of frequent urination, urgency, urinary incontinence or a combination of all these symptoms in patients with overactive bladder (OAB) . Fesoterodine is a new generation of tolterodine upgraded drug, with more stable PK characteristics. Two head-to-head studies have shown that fesoterodine 8mg is better than tolterodine 4mg in reducing the onset of UUI; at the same time, fesoterodine can reduce the adverse effects of urinary retention compared with tolterodine. Probability. In 2017, the global urinary incontinence drug treatment market was about US$3.4 billion, with a huge market capacity.
Hot Tags: fesoterodine fumarate api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Escitalopram Oxalate API, Bosentan API, Idebenone API, Edoxaban API, Temozolomide API, Salmeterol Xinafoate API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China