If you have any question, please feel free to email us. We will touch with you as soon as possible.
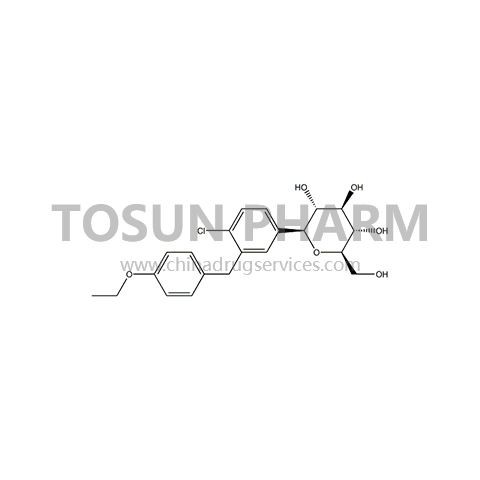
CAS:461432-26-8
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Dapagliflozin |
|---|---|
| Chinese name | 达格列净 |
| Cas Number | 461432-26-8 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Dapagliflozin is a new type of anti-diabetic drug jointly developed by Bristol-Myers Squibb and AstraZeneca. It was approved for marketing by the European Medicines Agency on November 12, 2012. It was the first approved market for treatment. Sodium-glucose cotransporter-2 (SGLT2) inhibitors for type 2 diabetes can be used as an important choice in the treatment of diabetes. Dapagliflozin selectively and strongly inhibits SGLT2, blocks the reabsorption of glucose by the proximal tubules, increases the excretion of glucose in the urine and reduces blood sugar. This hypoglycemic mechanism does not depend on the action of insulin. Clinical studies have shown that dapagliflozin as a single-drug therapy or combined therapy with metformin, glimepiride, pioglitazone, insulin and other drugs can effectively control blood sugar and reduce weight, and the risk of hypoglycemia is low. Dapagliflozin is also the first SGLT2 inhibitor to be proven effective for chronic kidney disease. The FDA granted Dapagliflozin Fast Track designation on September 16, 2019 to accelerate its development for delaying the progression of renal failure and preventing chronic kidney disease. The patient’s risk of cardiovascular and renal death.
Hot Tags: dapagliflozin api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Lifitegrast API, Ranolazine API, Oseltamivir Phosphate API, Lurasidone Hydrochloride API, Troxagliptin Succinate API, Apremilast API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China