If you have any question, please feel free to email us. We will touch with you as soon as possible.
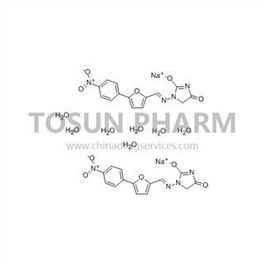
CAS:24868-20-0
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Dantrolene sodium |
|---|---|
| Chinese name | 丹曲林钠 |
| Cas Number | 24868-20-0 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Dantrolene sodium is a muscle relaxant created by Norwich Company of the United States in 1967. It has been included in the 12th edition of the Japanese Pharmacopoeia and is currently an orphan drug approved by the FDA for the treatment of malignant hyperthermia. Dantrolene sodium is different from general muscle relaxants. Its unique mechanism is to directly apply to skeletal muscles. It inhibits muscle contraction by inhibiting the release of calcium ions from the sarcoplasmic reticulum, thereby improving the patient's motor skills and increasing joint active and passive The range of activity reduces the symptoms related to the patient’s difficulty in exercise and promotes the recovery of functions. Compared with prednisolone and immunosuppressive agents such as methotrexate, dantrolene sodium can alleviate the sequelae effects of localized scleroderma and improve the quality of life of patients. The FDA granted dantrolene sodium orphan drug status in August 2013, which is expected to become a new clinical standard for the treatment of malignant hyperthermia. At present, in mainland China, the incidence of malignant hyperthermia is gradually increasing, but dantrolene sodium injection has not been approved for marketing, and there is no medicine available in hospitals and patients.
Hot Tags: dantrolene sodium api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Clofarabine API, Escitalopram Oxalate API, Adapalene API, Celecoxib API, Linagliptin API, Perindopril Tert butylamine Salt API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China