If you have any question, please feel free to email us. We will touch with you as soon as possible.
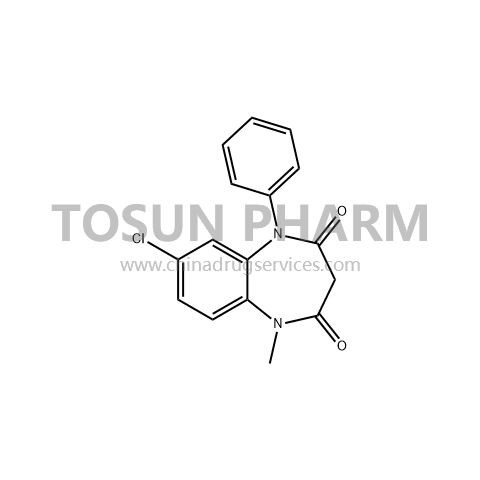
CAS:22316-47-8
Source:India
Qualifications:USDMF/CEP/-/-/-
| Name | Isodiazep |
|---|---|
| Chinese name | 氧异安定 |
| Cas Number | 22316-47-8 |
| Source | India |
| Qualifications | USDMF/CEP/-/-/- |
Clobazan was first synthesized by the Danish Lingbei company in the 1960s. It is a new type of benzodiazepine compound. In 2008, it obtained the orphan drug designation for the treatment of Dravet syndrome granted by the FDA. On October 21, 2011, the U.S. Food and Drug Administration (FDA) approved the clobazan tablets developed by Lundbeck LLC (Lundbeck Pharmaceuticals) pharmaceutical company, mainly for adults and children over two years old Lennox-Gastaut The adjuvant treatment of epileptic seizures in patients with Lundbeck syndrome (LGS, minor seizure variant) brought Lundbeck $380 million in revenue in 2016. Because LGS affects more than 200,000 people in the United States, it is approved as an orphan drug. Lin-German syndrome is a severe type of epilepsy, which is difficult to treat, and the patient may lose mobility. Therefore, the approval of this product is of great significance. The listing of Ba Zhan can provide treatment options for patients and their families affected by refractory epilepsy, and help patients and their families regain a normal and quality life.
Hot Tags: isodiazep api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Bosentan API, Linagliptin API, Tavaborol API, Gabapentin API, Lapatinib Ditosylate API, Teriflunomide API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China