If you have any question, please feel free to email us. We will touch with you as soon as possible.
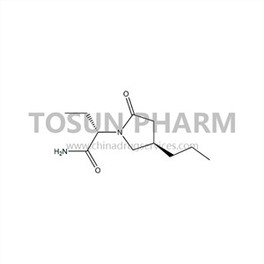
CAS:357336-20-0
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Bouracetam |
|---|---|
| Chinese name | 布瓦西坦 |
| Cas Number | 357336-20-0 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Buwaxitan is a new type of anti-epileptic drugs (AEDs) launched by the Belgian UCB company. It was approved by the US FDA in February 2016. It is a newer generation of anti-epileptic drugs than Levetiracetam. Buwaxitan is a selective, high-affinity synaptic vesicle protein 2A and levetiracetam analogue, with a small dose, highly permeable and after oral administration is quickly and almost completely Absorption characteristics, and suitable for medication for the elderly. Levetiracetam (Kaipura) has become a first-line drug because it can be used for the sole treatment of epilepsy, does not interact with other antiepileptic drugs, and is well tolerated. The structure of Buwaxitan is similar to Levetiracetam, and it exerts anti-epileptic effects by binding to synaptic vesicle protein 2A (SV2A). The binding force of Buwaxitan and SV2A is about 10 times that of Levetiracetam. Buwaxitan has the characteristics of high bioavailability and short peak time. Buvaracetam's good pharmacological activity, clinical efficacy and safety make it expected to become another blockbuster anti-epileptic drug after Levetiracetam. Buwaxitan was included in the first batch of national encouraged generic drugs list in 2019.
Hot Tags: bouracetam api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Solinasine Succinate API, Itraconazole Hydrochloride API, Pabxilib API, Vardenafil API, Teriflunomide API, Pregabalin API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China