If you have any question, please feel free to email us. We will touch with you as soon as possible.
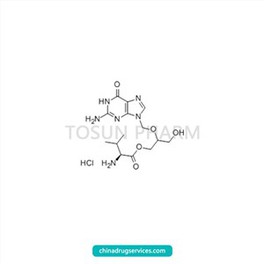
CAS:175865-59-5
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Valganciclovir hydrochloride |
|---|---|
| Chinese name | 盐酸缬更昔洛韦 |
| Cas Number | 175865-59-5 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Valganciclovir hydrochloride is a herpes virus DNA synthesis inhibitor, developed by the Swiss company Roche. It was first marketed as a prescription drug in Sweden and Finland in 2010. It was approved by the US FDA in March 2001 and was first marketed in the US in May 2001. Sales. Valganciclovir hydrochloride is a prodrug of ganciclovir and an active ganciclovir valine ester. After oral administration, it can be rapidly hydrolyzed into ganciclovir by phosphatase in intestinal and liver cells Its antiviral spectrum and mechanism of action are similar to ganciclovir, but its bioavailability is significantly higher than ganciclovir. Its oral absorption bioavailability is 62.4%, which is 10 times that of ganciclovir. , And the toxicity is greatly reduced. Clinical trials have shown that after two years of treatment with valganciclovir hydrochloride, 62% of cases are still alive, while the survival rate of cases not receiving valganciclovir is only 18%. Many studies at home and abroad have shown that Valganciclovir hydrochloride tablets have obvious pharmacological effects, novel mechanism of action, low toxicity, few adverse reactions, significant clinical efficacy, high patient compliance, and safe application.
Hot Tags: valganciclovir hydrochloride api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Pabxilib API, Pleasant API, Canagliflozin API, Linagliptin API, Dimethyl Fumarate API, Rasagiline Mesylate API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China