If you have any question, please feel free to email us. We will touch with you as soon as possible.
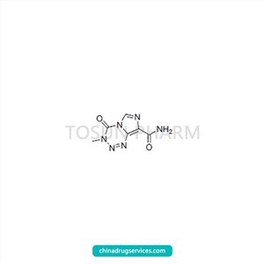
CAS:85622-93-1
Source:India
Qualifications:USDMF/CEP/-/-/-
| Name | Temozolomide |
|---|---|
| Chinese name | 替莫唑胺 |
| Cas Number | 85622-93-1 |
| Source | India |
| Qualifications | USDMF/CEP/-/-/- |
Temozolomide was developed by Schering-plough, and was approved by the FDA for listing in the United States on August 11, 1999. At the same time, it was marketed in many countries. This product is an alkylating agent of imidazotetrazine with anti-tumor activity. It can be used to treat newly diagnosed adult patients with glioblastoma multiforme. It can be used for combined radiotherapy and subsequent maintenance therapy. It is used clinically For the treatment of adult patients with refractory anaplastic astrocytoma and refractory glioblastoma multiforme. In addition, temozolomide has therapeutic activity on a variety of central nervous system cancers, including tumors that are resistant to carmustine. The product has less bone marrow suppression and is well tolerated. The injection was approved for marketing in the United States in 2009. Temozolomide for injection is currently on the market in many countries and regions such as the European Union, the United States and Japan. Only temozolomide capsules have been approved for marketing in China. Temozolomide has been included in the "List of Listed Drugs in China", and its terminal sales in medical institutions exceeded 1.8 billion yuan in 2017.
Hot Tags: temozolomide api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Dimethyl Fumarate API, Ranolazine API, Teriflunomide API, Gabapentin API, Ticagrelor API, Vinpocetine API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China