If you have any question, please feel free to email us. We will touch with you as soon as possible.
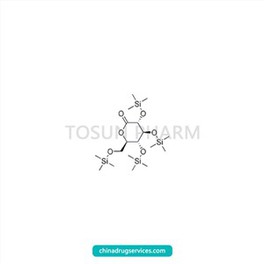
CAS:32384-65-9
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Canagliflozin |
|---|---|
| Chinese name | 卡格列净 |
| Cas Number | 32384-65-9 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Canagliflozin is the first SGLT2 inhibitor drug approved by the US FDA jointly developed by Tanabe Mitsubishi Pharmaceuticals and Johnson & Johnson. It is an oral hypoglycemic drug targeting type 2 sodium-glucose cotransporter (SGLT2). The target site and mechanism of SGLT-2 are different from the existing oral hypoglycemic drugs and antidiabetic drugs, and it has creatively opened up a new path for hypoglycemic. The mechanism of action of canagliflozin has nothing to do with insulin, so its combined use with any other diabetes treatment options (including insulin) can provide additional hypoglycemic effects. It can be used in combination with other oral hypoglycemic agents for patients with poor blood sugar control or insulin resistance under existing treatment schemes, and it can also be combined with insulin for patients with very low β-cell function and the existing oral drugs cannot work. In addition to good blood sugar control, canagliflozin has a more significant weight-loss effect, and compared with glimepiride, canagliflozin causes far fewer hypoglycemic events. In 2019, global sales of type 2 diabetes drugs were US$36 billion.
Hot Tags: canagliflozin api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Paliperidone API, Pregabalin API, Escitalopram Oxalate API, Fondaparinux API, Adapalene API, Temozolomide API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China