If you have any question, please feel free to email us. We will touch with you as soon as possible.
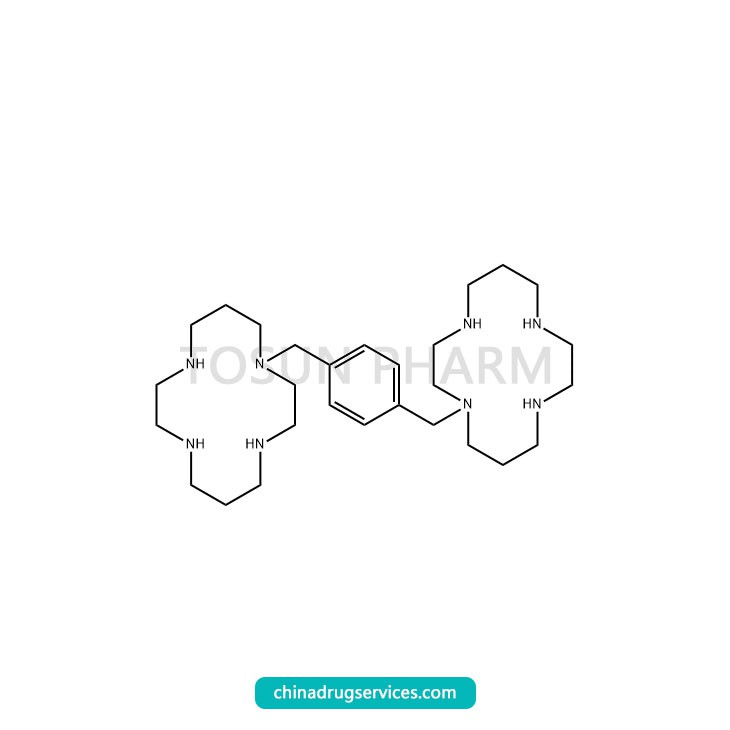
CAS:110078-46-1
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Pleasant |
|---|---|
| Chinese name | 普乐沙福 |
| Cas Number | 110078-46-1 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Plexifo is developed by Genzyme Pharmaceuticals, a subsidiary of Sanofi, as a mobilizer for autologous hematopoietic stem cell transplantation for patients with non-Hodgkin's lymphoma (NHL). It was first approved for marketing in the United States in 2008 and was awarded an orphan drug by the FDA Certification qualification. After being approved by the European Medicines Agency (EMA) in 2009, it has been used in more than 50 countries and regions around the world due to its excellent efficacy. The drug is a hematopoietic stem cell activator, and it can stimulate the proliferation and differentiation of hematopoietic stem cells into the blood circulation. A number of experimental studies have proved that under the combined action of granulocyte stimulating factor (G-CSF) or granule mononuclear cell stimulating factor (GM-CSF), Plexifa can make hematopoietic stem (progenitor) cells proliferate and release them into peripheral blood Significant increase in the number of peripheral blood CD34+ cell populations and hematopoietic progenitor cell colonies can significantly increase the success rate of autologous hematopoietic stem cell transplantation.
Hot Tags: pleasant api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Bivalrudine API, Bemeprost API, Pregabalin API, Pabxilib API, Teriflunomide API, Lapatinib Ditosylate API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China