If you have any question, please feel free to email us. We will touch with you as soon as possible.
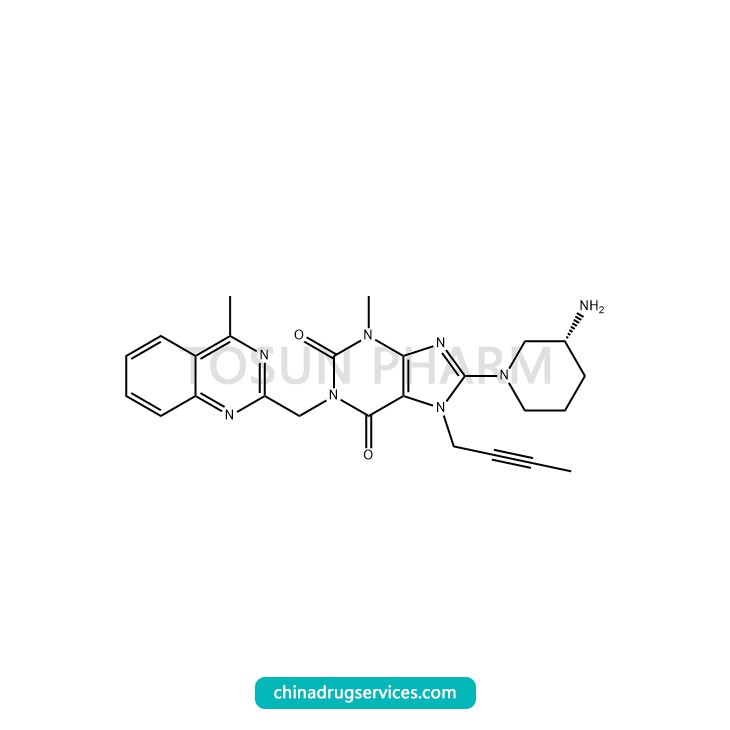
CAS:668270-12-0
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Linagliptin |
|---|---|
| Chinese name | 利格列汀 |
| Cas Number | 668270-12-0 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Linagliptin is an oral hypoglycemic drug developed by Boehringer Ingelheim, Germany. It was approved by the US FDA in May 2011 for marketing and was approved for marketing in China in April 2013. Linagliptin is a very active DPP-4 inhibitor with high selectivity, long-acting and oral effectiveness. It can prolong the half-life of active glucagon-like polypeptide-1 (GLP-1) in the body and exert its effectiveness. Glucose-dependent insulin-promoting effect, and therefore increase plasma insulin levels, lower blood sugar, while having good safety and tolerability; compared with other DPP-4 inhibitors, Linagliptin has excellent renal safety, And can effectively reduce the advantages of glycosylated hemoglobin. Linagliptin has the same effect as the commonly used sulfonylurea drug glimepiride, and the hypoglycemic effect can be maintained for 104 weeks. Compared with glimepiride, linagliptin does not increase cardiovascular risk, and the risk of hypoglycemia is small. The scale of China's diabetes market is RMB 40 billion, of which oral drugs account for 56%. Linagliptin is expected to become a new growth point in the sales of DPP-4 inhibitors.
Hot Tags: linagliptin api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Tetrabenazine API, Pabxilib API, Deoxycholic Acid API, Adapalene API, Salmeterol Xinafoate API, Tippyridine Hydrochloride API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China