If you have any question, please feel free to email us. We will touch with you as soon as possible.
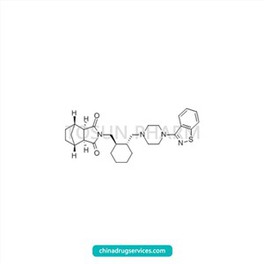
CAS:367514-88-3
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Lurasidone Hydrochloride |
|---|---|
| Chinese name | 盐酸鲁拉西酮 |
| Cas Number | 367514-88-3 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
On January 24, 2019, the antipsychotic drug lurasidone was approved for marketing in China. Lurasidone (Rosuda) was developed by Japan's Sumitomo Pharmaceuticals and belongs to the atypical antipsychotic class. Because of its high affinity for 5-HT2A receptor and dopamine D2 receptor, it is approved for schizophrenia. Treatment of patients. The FDA has also approved the extended indication of lurasidone for the treatment of major depressive episodes associated with bipolar I disorder in children and adolescents aged 10-17 years and for the treatment of bipolar adults as a single agent or in combination with lithium/valproate Depression. As an atypical antipsychotic drug, Lurasidone has a unique mechanism of action, precise curative effect, high safety, less impact on body weight and metabolic indicators, once a day, and convenient administration compared with similar drugs. The main patent of lurasidone hydrochloride in China has expired or withdrawn. There should be no key obstacles. In the next few years, this product will be a popular product to be copied in the field of indications.
Hot Tags: lurasidone hydrochloride api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Apremilast API, Solinasine Succinate API, Ulipristal Acetate API, Pleasant API, Pabxilib API, Deoxycholic Acid API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China