If you have any question, please feel free to email us. We will touch with you as soon as possible.
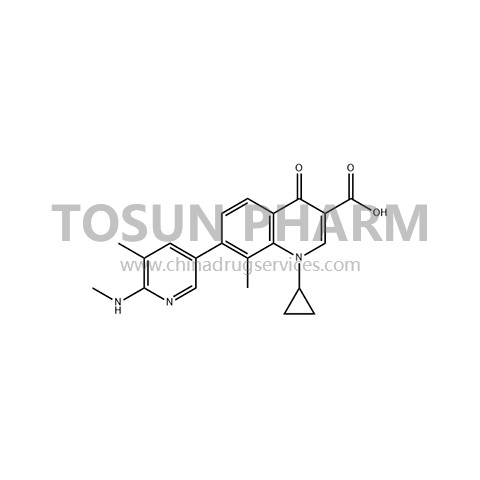
CAS:245765-41-7
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Ozefloxacin |
|---|---|
| Chinese name | 奥泽沙星 |
| Cas Number | 245765-41-7 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Ozefloxacin is a quinolone antibacterial drug developed by Ferrer Internacional Co., Ltd. of Spain. It was purchased by Medimetriks Pharmaceuticals of the United States and obtained the exclusive commercialization rights in the United States. It was approved by the Ministry of Health, Labour and Welfare of Japan in September 2015, becoming the first new molecule approved in the world. The entity was approved by the US FDA for listing on December 11, 2017. Ozefloxacin's mechanism of action is to inhibit bacterial DNA replicase, DNA gyrase A and topoisomerase IV. Ozefloxacin is the third non-fluoroquinolone antibacterial drug approved in the world after nanofloxacin and garefloxacin. In clinical trials, Ozefloxacin was used to treat skin and soft tissue infections, and the efficacy was better than mupirocin, with no obvious adverse reactions. Ozefloxacin has significantly stronger inhibitory effects on MRSA, methicillin-sensitive Staphylococcus aureus (MSSA), and ofloxacin-resistant Staphylococcus aureus than nafloxacin, ofloxacin, levofloxacin, clindamycin , Erythromycin and gentamicin. Ozefloxacin has strong bactericidal activity and can become a new quinolone drug that shortens the course of treatment and has good clinical application prospects.
Hot Tags: ozefloxacin api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Ranolazine API, Edoxaban API, Brinzolamide API, Lifitegrast API, Pleasant API, Dimethyl Fumarate API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China