If you have any question, please feel free to email us. We will touch with you as soon as possible.
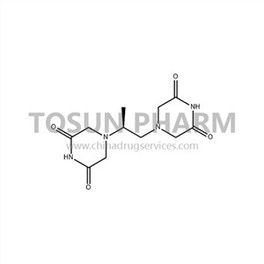
CAS:24584-09-6
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Right Rezoo |
|---|---|
| Chinese name | 右雷佐生 |
| Cas Number | 24584-09-6 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Rezoxan was developed by Chiron Corporation of the United States. It was first listed in Italy in 1992 and was approved by the FDA in July 1995. Afterwards, it has been listed in many countries such as Europe, Asia and Africa. Dexrazoxane is clinically suitable for the prevention of cardiotoxicity induced by anthracyclines. The marketed dosage form is injection, the specifications are 250mg and 500mg, and the diluent is 0.167mol/l sodium lactate 25ml and 50ml respectively. Dexrazoxane is a d-isomer of racemic razoxane and a lipophilic derivative of the chelating agent ethylenediaminetetraacetic acid (EDTA). It can quickly penetrate cell membranes and reduce doxorubicin etc. The cardiotoxicity of anthracycline anti-tumor antibiotics, dexrazoxane is hydrolyzed into TCRE-198 in the cell, and then chelated with the iron in the cell, so that the trivalent iron ion is compounded with doxorubicin and other anthracycline anti-tumor drugs It is effective to prevent the formation of free radicals by reducing substances. Dexrazoxane can also inhibit the cytotoxicity of topoisomerase II, and can enhance or antagonize the cytotoxicity of certain antitumor drugs in animal models. Dexrazoxane is currently the only drug that is clinically approved to prevent the cardiotoxicity of anthracyclines. The current clinical trials in European and American countries show that the drug is expected to become the standard compatibility of anthracycline antitumor drugs. Although dexrazoxane is currently mainly used to prevent cardiotoxicity induced by anthracyclines, the drug has the potential to regulate the activity of topoisomerase II and cellular iron metabolism, and will be used in cancer treatment, immunology, infectious diseases, etc. Aspects of application will likely become the focus.
Hot Tags: right rezoo api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Aprepitant Aprepitant API, Dimethyl Fumarate API, Solinasine Succinate API, Tavaborol API, Active Pharmaceutical Ingredient, Idebenone API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China