If you have any question, please feel free to email us. We will touch with you as soon as possible.
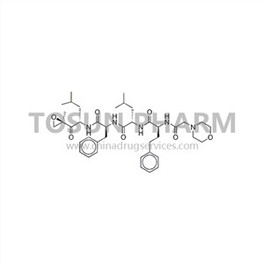
CAS:868540-17-4
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Carfilzomib |
|---|---|
| Chinese name | 卡非佐米 |
| Cas Number | 868540-17-4 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
The original research is Amgen, and it was approved by the FDA in July 2012. It belongs to the proteasome inhibitor (PI) together with bortezomib for the treatment of relapsed/refractory multiple myeloma (MM). Compared with bortezomib, carfilzomib has stronger proteasome inhibitory activity and fewer off-target effects. As a new generation of highly selective irreversible proteasome blocking drugs, multiple clinical studies have confirmed that carfilzomib alone or in combination with other drugs has a strong anti-MM effect with little toxicity, especially the incidence of peripheral neuropathy. Low, good patient tolerance, high safety, and long-term clinical use. The industry predicts that it may replace bortezomib, and the market prospects are promising.
Hot Tags: carfilzomib api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Bivalrudine API, Lifitegrast API, Brinzolamide API, Troxagliptin Succinate API, Ticagrelor API, Lurasidone Hydrochloride API
Tel:+86-020-61855200-673
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China