If you have any question, please feel free to email us. We will touch with you as soon as possible.
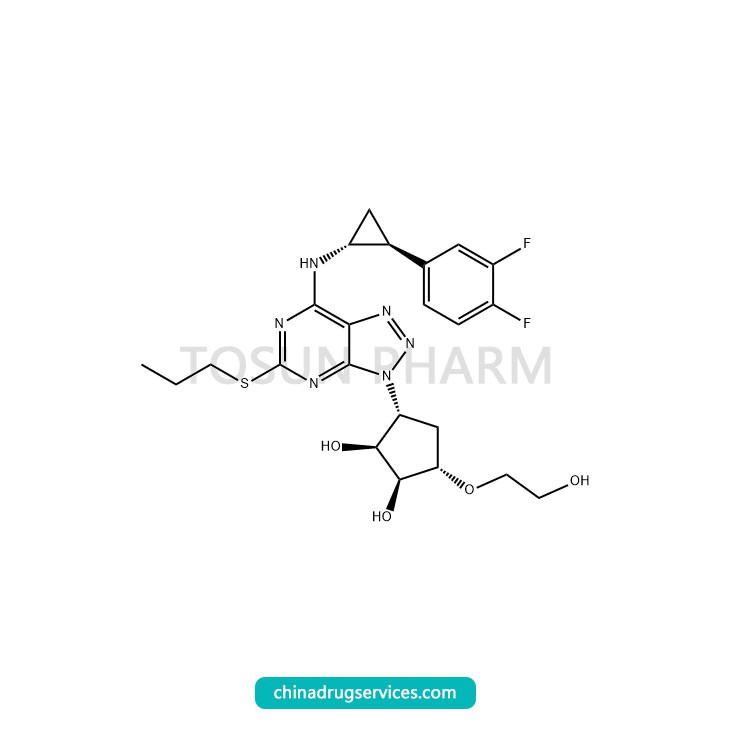
CAS:274693-27-5
Source:India
Qualifications:USDMF/-/-/-/-
| Name | Ticagrelor |
|---|---|
| Chinese name | 替格瑞洛 |
| Cas Number | 274693-27-5 |
| Source | India |
| Qualifications | USDMF/-/-/-/- |
Ticagrelor is a new and selective small molecule anticoagulant, which was approved by the FDA in July 2011. It is the first reversible, direct-acting, orally administered novel P2Y12 receptor antagonist, used to reduce cardiovascular deaths and heart attacks in patients with acute coronary syndrome (ACS), and entered China in December 2012 Market, entered China's National Medical Insurance Catalog in 2017. At present, ticagrelor has been recommended by many international treatment guidelines for the first-line treatment of ACS patients, and its clinical status has become more prominent, becoming a blockbuster product with sales exceeding 1 billion.
Hot Tags: ticagrelor api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock, Escitalopram Oxalate API, Tetrabenazine API, Paliperidone API, Pabxilib API, Lapatinib Ditosylate API, Bemeprost API
Tel:+86-020-61855200-902
Fax:+86-020-66392525
Email:info@upharm.cn
Address:12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China